Coexistence of a two-states organization for a cell-penetrating peptide in lipid bilayer
- PMID: 16199494
- PMCID: PMC1366994
- DOI: 10.1529/biophysj.105.061697
Coexistence of a two-states organization for a cell-penetrating peptide in lipid bilayer
Abstract
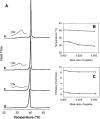
Primary amphipathic cell-penetrating peptides transport cargoes across cell membranes with high efficiency and low lytic activity. These primary amphipathic peptides were previously shown to form aggregates or supramolecular structures in mixed lipid-peptide monolayers, but their behavior in lipid bilayers remains to be characterized. Using atomic force microscopy, we have examined the interactions of P(alpha), a primary amphipathic cell-penetrating peptide which remains alpha-helical whatever the environment, with dipalmitoylphosphatidylcholine (DPPC) bilayers. Addition of P(alpha) at concentrations up to 5 mol % markedly modified the supported bilayers topography. Long and thin filaments lying flat at the membrane surface coexisted with deeply embedded peptides which induced a local thinning of the bilayer. On the other hand, addition of P(alpha) only exerted very limited effects on the corresponding liposome's bilayer physical state, as estimated from differential scanning calorimetry and diphenylhexatriene fluorescence anisotropy experiments. The use of a gel-fluid phase separated supported bilayers made of a dioleoylphosphatidylcholine/dipalmitoylphosphatidylcholine mixture confirmed both the existence of long filaments, which at low peptide concentration were preferentially localized in the fluid phase domains and the membrane disorganizing effects of 5 mol % P(alpha). The simultaneous two-states organization of P(alpha), at the membrane surface and deeply embedded in the bilayer, may be involved in the transmembrane carrier function of this primary amphipathic peptide.
Figures






References
-
- Lindgren, M., M. Hallbrink, A. Prochiantz, and U. Langel. 2000. Cell-penetrating peptides. Trends Pharmacol. Sci. 21:99–103. - PubMed
-
- Prochiantz, A. 1996. Getting hydrophilic compounds into cells: lessons from homeopeptides. Curr. Opin. Neurobiol. 6:629–634. - PubMed
-
- Prochiantz, A. 2000. Messenger proteins: homeoproteins, TAT and others. Curr. Opin. Cell Biol. 12:400–406. - PubMed
-
- Dietz, G. P., and M. Bahr. 2004. Delivery of bioactive molecules into the cell: the Trojan horse approach. Mol. Cell. Neurosci. 27:85–131. - PubMed
-
- Drin, G., S. Cottin, E. Blanc, A. R. Rees, and J. Temsamani. 2003. Studies on the internalization mechanism of cationic cell-penetrating peptides. J. Biol. Chem. 278:31192–31201. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

