Interactions between charged polypeptides and nonionic surfactants
- PMID: 16199501
- PMCID: PMC1366987
- DOI: 10.1529/biophysj.105.065342
Interactions between charged polypeptides and nonionic surfactants
Abstract
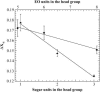
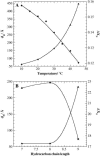
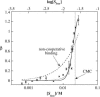
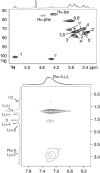
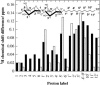
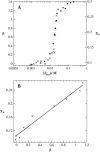
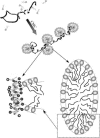
The influence of molecular characteristics on the mutual interaction between peptides and nonionic surfactants has been investigated by studying the effects of surfactants on amphiphilic, random copolymers of alpha-L-amino acids containing lysine residues as the hydrophilic parts. The hydrophobic residues were either phenylalanine or tyrosine. The peptide-surfactant interactions were studied by means of circular dichroism spectroscopy and binding isotherms, as well as by 1D and 2D NMR. The binding of surfactant to the peptides was found to be a cooperative process, appearing at surfactant concentrations just below the critical micellar concentration. However, a certain degree of peptide hydrophobicity is necessary to obtain an interaction with nonionic surfactant. When this prerequisite is fulfilled, the peptide mainly interacts with self-assembled, micelle-like surfactant aggregates formed onto the peptide chain. Therefore, the peptide-surfactant complex is best described in terms of a necklace model, with the peptide interacting primarily with the palisade region of the micelles via its hydrophobic side chains. The interaction yields an increased amount of alpha-helix conformation in the peptide. Surfactants that combine small headgroups with a propensity to form small, nearly spherical micelles were shown to give the largest increase in alpha-helix content.
Figures







References
-
- Kuroda, Y., Y. Maeda, S. Sawa, K. Shibata, K. Miyamoto, and T. Nakagawa. 2003. Effects of detergent on the secondary structures of prion protein peptides as studied by CD spectroscopy. J. Pept. Sci. 9:212–220. - PubMed
-
- Wieprecht, T., M. Beyermann, and J. Seelig. 1999. Binding of antibacterial magainin peptides to electrical neutral membranes: thermodynamics and structure. Biochemistry. 38:10377–10387. - PubMed
-
- Wieprecht, T., M. Beyermann, and J. Seelig. 2002. Thermodynamics of the coil-α-helix transition of amphipathic peptides in a membrande environment: the role of vesicle curvature. Biophys. Chem. 96:191–201. - PubMed
-
- Wieprecht, T., S. Rothemund, M. Bienert, and E. Krause. 2001. Role of helix formation for the retention of peptides in reversed-phase high-performance liquid chromatography. J. Chromatogr. A. 912:1–12. - PubMed
-
- Wieprecht, T., O. Apostolov, M. Beyermann, and J. Seelig. 2000. Interaction of a mitochondrial presequence with lipid membranes: role of helix formation for membrane binding and perturbation. Biochemistry. 39:15297–15305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

