Distinct constrictive processes, separated in time and space, divide caulobacter inner and outer membranes
- PMID: 16199556
- PMCID: PMC1251605
- DOI: 10.1128/JB.187.20.6874-6882.2005
Distinct constrictive processes, separated in time and space, divide caulobacter inner and outer membranes
Abstract
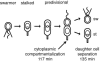
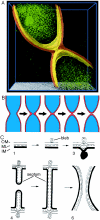
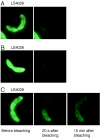
Cryoelectron microscope tomography (cryoEM) and a fluorescence loss in photobleaching (FLIP) assay were used to characterize progression of the terminal stages of Caulobacter crescentus cell division. Tomographic cryoEM images of the cell division site show separate constrictive processes closing first the inner membrane (IM) and then the outer membrane (OM) in a manner distinctly different from that of septum-forming bacteria. FLIP experiments had previously shown cytoplasmic compartmentalization (when cytoplasmic proteins can no longer diffuse between the two nascent progeny cell compartments) occurring 18 min before daughter cell separation in a 135-min cell cycle so the two constrictive processes are separated in both time and space. In the very latest stages of both IM and OM constriction, short membrane tether structures are observed. The smallest observed pre-fission tethers were 60 nm in diameter for both the inner and outer membranes. Here, we also used FLIP experiments to show that both membrane-bound and periplasmic fluorescent proteins diffuse freely through the FtsZ ring during most of the constriction procession.
Figures



Comment in
-
High-resolution anatomy of a progressively pinching cell division.J Bacteriol. 2005 Oct;187(20):6867-9. doi: 10.1128/JB.187.20.6867-6869.2005. J Bacteriol. 2005. PMID: 16199554 Free PMC article. Review. No abstract available.
References
-
- Addinall, S. G., and B. Holland. 2002. The tubulin ancestor, FtsZ, draughtsman, designer and driving force for bacterial cytokinesis. J. Mol. Biol. 318:219-236. - PubMed
-
- Addinall, S. G., and J. Lutkenhaus. 1996. FtsZ-spirals and -arcs determine the shape of the invaginating septa in some mutants of Escherichia coli. Mol. Microbiol. 22:231-237. - PubMed
-
- Ausmees, N., and C. Jacobs-Wagner. 2003. Spatial and temporal control of differentiation and cell cycle progression in Caulobacter crescentus. Annu. Rev. Microbiol. 57:225-247. - PubMed
-
- Bi, E. F., and J. Lutkenhaus. FtsZ ring structure associated with division in Escherichia coli. Nature 354:161-164, 1991. - PubMed

