The Mycobacterium tuberculosis extracytoplasmic-function sigma factor SigL regulates polyketide synthases and secreted or membrane proteins and is required for virulence
- PMID: 16199577
- PMCID: PMC1251616
- DOI: 10.1128/JB.187.20.7062-7071.2005
The Mycobacterium tuberculosis extracytoplasmic-function sigma factor SigL regulates polyketide synthases and secreted or membrane proteins and is required for virulence
Abstract
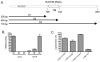
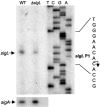
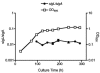
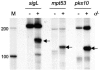
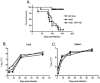
Mycobacterium tuberculosis sigL encodes an extracytoplasmic function (ECF) sigma factor and is adjacent to a gene for a membrane protein (Rv0736) that contains a conserved HXXXCXXC sequence. This motif is found in anti-sigma factors that regulate several ECF sigma factors, including those that control oxidative stress responses. In this work, SigL and Rv0736 were found to be cotranscribed, and the intracellular domain of Rv0736 was shown to interact specifically with SigL, suggesting that Rv0736 may encode an anti-sigma factor of SigL. An M. tuberculosis sigL mutant was not more susceptible than the parental strain to several oxidative and nitrosative stresses, and sigL expression was not increased in response to these stresses. In vivo, sigL is expressed from a weak SigL-independent promoter and also from a second SigL-dependent promoter. To identify SigL-regulated genes, sigL was overexpressed and microarray analysis of global transcription was performed. Four small operons, sigL (Rv0735)-Rv0736, mpt53 (Rv2878c)-Rv2877c, pks10 (Rv1660)-pks7 (Rv1661), and Rv1139c-Rv1138c, were among the most highly upregulated genes in the sigL-overexpressing strain. SigL-dependent transcription start sites of these operons were mapped, and the consensus promoter sequences TGAACC in the -35 region and CGTgtc in the -10 region were identified. In vitro, purified SigL specifically initiated transcription from the promoters of sigL, mpt53, and pks10. Additional genes, including four PE_PGRS genes, appear to be regulated indirectly by SigL. In an in vivo murine infection model, the sigL mutant strain showed marked attenuation, indicating that the sigL regulon is important in M. tuberculosis pathogenesis.
Figures
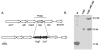


Similar articles
-
Posttranslational regulation of Mycobacterium tuberculosis extracytoplasmic-function sigma factor sigma L and roles in virulence and in global regulation of gene expression.Infect Immun. 2006 Apr;74(4):2457-61. doi: 10.1128/IAI.74.4.2457-2461.2006. Infect Immun. 2006. PMID: 16552079 Free PMC article.
-
Mycobacterium tuberculosis ECF sigma factor sigC is required for lethality in mice and for the conditional expression of a defined gene set.Mol Microbiol. 2004 Apr;52(1):25-38. doi: 10.1111/j.1365-2958.2003.03958.x. Mol Microbiol. 2004. PMID: 15049808
-
Transcription regulation by the Mycobacterium tuberculosis alternative sigma factor SigD and its role in virulence.J Bacteriol. 2004 Oct;186(19):6605-16. doi: 10.1128/JB.186.19.6605-6616.2004. J Bacteriol. 2004. PMID: 15375142 Free PMC article.
-
The extracytoplasmic function sigma factors: role in bacterial pathogenesis.Infect Genet Evol. 2004 Dec;4(4):301-8. doi: 10.1016/j.meegid.2004.04.003. Infect Genet Evol. 2004. PMID: 15374527 Review.
-
The sigma factors of Mycobacterium tuberculosis: regulation of the regulators.FEBS J. 2010 Feb;277(3):605-26. doi: 10.1111/j.1742-4658.2009.07479.x. Epub 2009 Nov 27. FEBS J. 2010. PMID: 19951358 Review.
Cited by
-
Mycobacterium tuberculosis Serine/Threonine Protein Kinases.Microbiol Spectr. 2014 Oct;2(5):10.1128/microbiolspec.MGM2-0006-2013. doi: 10.1128/microbiolspec.MGM2-0006-2013. Microbiol Spectr. 2014. PMID: 25429354 Free PMC article. Review.
-
The rv1184c locus encodes Chp2, an acyltransferase in Mycobacterium tuberculosis polyacyltrehalose lipid biosynthesis.J Bacteriol. 2015 Jan 1;197(1):201-10. doi: 10.1128/JB.02015-14. Epub 2014 Oct 20. J Bacteriol. 2015. PMID: 25331437 Free PMC article.
-
Characterization of the Mycobacterium tuberculosis sigma factor SigM by assessment of virulence and identification of SigM-dependent genes.Infect Immun. 2007 Jan;75(1):452-61. doi: 10.1128/IAI.01395-06. Epub 2006 Nov 6. Infect Immun. 2007. PMID: 17088352 Free PMC article.
-
Mycobacterium tuberculosis modulates its cell surface via an oligopeptide permease (Opp) transport system.FASEB J. 2009 Dec;23(12):4091-104. doi: 10.1096/fj.09-132407. Epub 2009 Aug 11. FASEB J. 2009. PMID: 19671666 Free PMC article.
-
Delineating transcriptional crosstalk between Mycobacterium avium subsp. paratuberculosis and human THP-1 cells at the early stage of infection via dual RNA-seq analysis.Vet Res. 2022 Sep 13;53(1):71. doi: 10.1186/s13567-022-01089-y. Vet Res. 2022. PMID: 36100945 Free PMC article.
References
-
- Bae, J. B., J. H. Park, M. Y. Hahn, M. S. Kim, and J. H. Roe. 2004. Redox-dependent changes in RsrA, an anti-sigma factor in Streptomyces coelicolor: zinc release and disulfide bond formation. J. Mol. Biol. 335:425-435. - PubMed
-
- Bardarov, S., S. Bardarov, Jr., M. S. Pavelka, Jr., V. Sambandamurthy, M. Larsen, J. Tufariello, J. Chan, G. Hatfull, and W. R. Jacobs, Jr. 2002. Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology 148:3007-3017. - PubMed
-
- Beckett, C. S., J. A. Loughman, K. A. Karberg, G. M. Donato, W. E. Goldman, and R. G. Kranz. 2000. Four genes are required for the system II cytochrome c biogenesis pathway in Bordetella pertussis, a unique bacterial model. Mol. Microbiol. 38:465-481. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

