Rejection of impassable substrates by Yersinia type III secretion machines
- PMID: 16199580
- PMCID: PMC1251613
- DOI: 10.1128/JB.187.20.7090-7102.2005
Rejection of impassable substrates by Yersinia type III secretion machines
Abstract
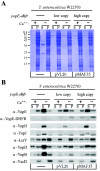
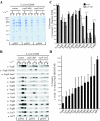
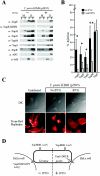
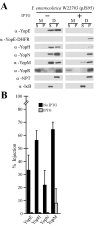
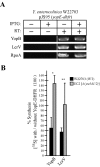
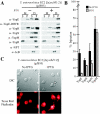
Type III machines of pathogenic Yersinia spp. transport Yop proteins across the bacterial envelope into host cells. Translational fusions of yopE to the dihydrofolate reductase gene (dhfr) or the beta-galactosidase gene (lacZ) generate hybrid proteins that block type III injection of Yop proteins into host cells, consistent with the canonical view that impassable DHFR and LacZ hybrids jam secretion machines. Mutations in repressors of posttranscriptional gene regulation, Yersinia enterocolitica yscM1 and yscM2 as well as Yersinia pestis lcrQ, relieve the YopE-DHFR-imposed blockade and restore type III injection into host cells. Genetic suppression of the type III blockade does not, however, promote YopE-DHFR secretion. A model is proposed whereby rejection of YopE-DHFR from the secretion pathway inhibits type III gene expression.
Figures





Similar articles
-
SycE allows secretion of YopE-DHFR hybrids by the Yersinia enterocolitica type III Ysc system.Mol Microbiol. 2002 Nov;46(4):1183-97. doi: 10.1046/j.1365-2958.2002.03241.x. Mol Microbiol. 2002. PMID: 12421321
-
Yersinia enterocolitica type III secretion: yscM1 and yscM2 regulate yop gene expression by a posttranscriptional mechanism that targets the 5' untranslated region of yop mRNA.J Bacteriol. 2002 Nov;184(21):5880-93. doi: 10.1128/JB.184.21.5880-5893.2002. J Bacteriol. 2002. PMID: 12374821 Free PMC article.
-
Yersinia enterocolitica type III secretion: evidence for the ability to transport proteins that are folded prior to secretion.BMC Microbiol. 2004 Jul 12;4:27. doi: 10.1186/1471-2180-4-27. BMC Microbiol. 2004. PMID: 15248901 Free PMC article.
-
Role of a plasmid in the pathogenicity of Yersinia species.Curr Top Microbiol Immunol. 1985;118:29-51. doi: 10.1007/978-3-642-70586-1_3. Curr Top Microbiol Immunol. 1985. PMID: 3902382 Review. No abstract available.
-
Chromosomal virulence factors of Yersinia: an update.Contrib Microbiol Immunol. 1995;13:218-24. Contrib Microbiol Immunol. 1995. PMID: 8833839 Review. No abstract available.
Cited by
-
Translational regulation of Yersinia enterocolitica mRNA encoding a type III secretion substrate.J Biol Chem. 2013 Dec 6;288(49):35478-88. doi: 10.1074/jbc.M113.504811. Epub 2013 Oct 24. J Biol Chem. 2013. PMID: 24158443 Free PMC article.
-
Remembering Malcolm J. Casadaban.J Bacteriol. 2010 Sep;192(17):4261-3. doi: 10.1128/JB.00484-10. Epub 2010 May 28. J Bacteriol. 2010. PMID: 20511498 Free PMC article.
-
Recruitment of heterologous substrates by bacterial secretion systems for transkingdom translocation.Front Cell Infect Microbiol. 2023 Mar 6;13:1146000. doi: 10.3389/fcimb.2023.1146000. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36949816 Free PMC article. Review.
-
RfaL is required for Yersinia pestis type III secretion and virulence.Infect Immun. 2013 Apr;81(4):1186-97. doi: 10.1128/IAI.01417-12. Epub 2013 Jan 28. Infect Immun. 2013. PMID: 23357388 Free PMC article.
-
Characterization of the Yersinia enterocolitica type III secretion ATPase YscN and its regulator, YscL.J Bacteriol. 2006 May;188(10):3525-34. doi: 10.1128/JB.188.10.3525-3534.2006. J Bacteriol. 2006. PMID: 16672607 Free PMC article.
References
-
- Agrain, C., I. Callebaut, L. Journet, I. Sorg, C. Paroz, L. J. Mota, and G. R. Cornelis. 2005. Characterization of a type III secretion substrate specificity switch (T3S4) domain in YscP from Yersinia enterocolitica. Mol. Microbiol. 56:54-67. - PubMed
-
- Amann, E., J. Brosius, and M. Ptashne. 1983. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene 25:167-178. - PubMed
-
- Anderson, D. M., and O. Schneewind. 1997. An mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science 278:1140-1143. - PubMed
-
- Anderson, D. M., and O. Schneewind. 1999. Yersinia enterocolitica type III secretion: an mRNA signal that couples translation and secretion of YopQ. Mol. Microbiol. 31:1139-1148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

