Ablation of MEKK4 kinase activity causes neurulation and skeletal patterning defects in the mouse embryo
- PMID: 16199873
- PMCID: PMC1265780
- DOI: 10.1128/MCB.25.20.8948-8959.2005
Ablation of MEKK4 kinase activity causes neurulation and skeletal patterning defects in the mouse embryo
Abstract
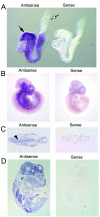
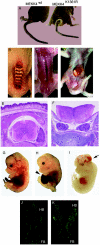
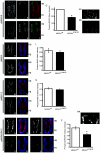
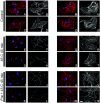
Skeletal disorders and neural tube closure defects represent clinically significant human malformations. The signaling networks regulating normal skeletal patterning and neurulation are largely unknown. Targeted mutation of the active site lysine of MEK kinase 4 (MEKK4) produces a kinase-inactive MEKK4 protein (MEKK4(K1361R)). Embryos homozygous for this mutation die at birth as a result of skeletal malformations and neural tube defects. Hindbrains of exencephalic MEKK4(K1361R) embryos show a striking increase in neuroepithelial cell apoptosis and a dramatic loss of phosphorylation of MKK3 and -6, mitogen-activated protein kinase kinases (MKKs) regulated by MEKK4 in the p38 pathway. Phosphorylation of MAPK-activated protein kinase 2, a p38 substrate, is also inhibited, demonstrating a loss of p38 activity in MEKK4(K1361R) embryos. In contrast, the MEK1/2-extracellular signal-regulated kinase 1 (ERK1)/ERK2 and MKK4-Jun N-terminal protein kinase pathways were unaffected. The p38 pathway has been shown to regulate the phosphorylation and expression of the small heat shock protein HSP27. Compared to the wild type, MEKK4(K1361R) fibroblasts showed significantly reduced phosphorylation of p38 and HSP27, with a corresponding heat shock-induced instability of the actin cytoskeleton. Together, these data demonstrate MEKK4 regulation of p38 and that substrates downstream of p38 control cellular homeostasis. The findings are the first demonstration that MEKK4-regulated p38 activity is critical for neurulation.
Figures



References
-
- Adams, R. H., A. Porras, G. Alonso, M. Jones, K. Vintersten, S. Panelli, A. Valladares, L. Perez, R. Klein, and A. R. Nebreda. 2000. Essential role of p38α MAP kinase in placental but not embryonic cardiovascular development. Mol. Cell 6:109-116. - PubMed
-
- Bhasin, N., E. Kernick, X. Luo, H. E. Seidel, E. R. Weiss, and J. M. Lauder. 2004. Differential regulation of chondrogenic differentiation by the serotonin 2B receptor and retinoic acid in the embryonic mouse hindlimb. Dev. Dyn. 230:201-209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
