The condensin I subunit Barren/CAP-H is essential for the structural integrity of centromeric heterochromatin during mitosis
- PMID: 16199875
- PMCID: PMC1265781
- DOI: 10.1128/MCB.25.20.8971-8984.2005
The condensin I subunit Barren/CAP-H is essential for the structural integrity of centromeric heterochromatin during mitosis
Abstract
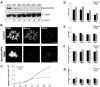
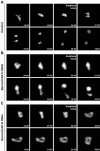
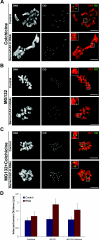
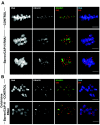
During cell division, chromatin undergoes structural changes essential to ensure faithful segregation of the genome. Condensins, abundant components of mitotic chromosomes, are known to form two different complexes, condensins I and II. To further examine the role of condensin I in chromosome structure and in particular in centromere organization, we depleted from S2 cells the Drosophila CAP-H homologue Barren, a subunit exclusively associated with condensin I. In the absence of Barren/CAP-H the condensin core subunits DmSMC4/2 still associate with chromatin, while the other condensin I non-structural maintenance of chromosomes family proteins do not. Immunofluorescence and in vivo analysis of Barren/CAP-H-depleted cells showed that mitotic chromosomes are able to condense but fail to resolve sister chromatids. Additionally, Barren/CAP-H-depleted cells show chromosome congression defects that do not appear to be due to abnormal kinetochore-microtubule interaction. Instead, the centromeric and pericentromeric heterochromatin of Barren/CAP-H-depleted chromosomes shows structural problems. After bipolar attachment, the centromeric heterochromatin organized in the absence of Barren/CAP-H cannot withstand the forces exerted by the mitotic spindle and undergoes irreversible distortion. Taken together, our data suggest that the condensin I complex is required not only to promote sister chromatid resolution but also to maintain the structural integrity of centromeric heterochromatin during mitosis.
Figures



References
-
- Almagro, S., D. Riveline, T. Hirano, B. Houchmandzadeh, and S. Dimitrov. 2004. The mitotic chromosome is an assembly of rigid elastic axes organized by structural maintenance of chromosomes (SMC) proteins and surrounded by a soft chromatin envelope. J. Biol. Chem. 279:5118-5126. - PubMed
-
- Aono, N., T. Sutani, T. Tomonaga, S. Mochida, and M. Yanagida. 2002. Cnd2 has dual roles in mitotic condensation and interphase. Nature 417:197-202. - PubMed
-
- Bhat, M. A., A. V. Philp, D. M. Glover, and H. J. Bellen. 1996. Chromatid segregation at anaphase requires the barren product, a novel chromosome-associated protein that interacts with topoisomerase II. Cell 87:1103-1114. - PubMed
-
- Bickmore, W. A., and K. Oghene. 1996. Visualizing the spatial relationships between defined DNA sequences and the axial region of extracted metaphase chromosomes. Cell 84:95-104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
