The Xenopus ELAV protein ElrB represses Vg1 mRNA translation during oogenesis
- PMID: 16199879
- PMCID: PMC1265794
- DOI: 10.1128/MCB.25.20.9028-9039.2005
The Xenopus ELAV protein ElrB represses Vg1 mRNA translation during oogenesis
Abstract
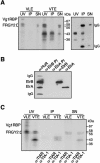
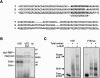
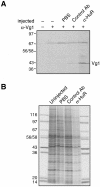
Xenopus laevis Vg1 mRNA undergoes both localization and translational control during oogenesis. We previously characterized a 250-nucleotide AU-rich element, the Vg1 translation element (VTE), in the 3'-untranslated region (UTR) of this mRNA that is responsible for the translational repression. UV-cross-linking and immunoprecipitation experiments, described here, revealed that the known AU-rich element binding proteins, ElrA and ElrB, and TIA-1 and TIAR interact with the VTE. The levels of these proteins during oogenesis are most consistent with a possible role for ElrB in the translational control of Vg1 mRNA, and ElrB, in contrast to TIA-1 and TIAR, is present in large RNP complexes. Immunodepletion of TIA-1 and TIAR from Xenopus translation extract confirmed that these proteins are not involved in the translational repression. Mutagenesis of a potential ElrB binding site destroyed the ability of the VTE to bind ElrB and also abolished translational repression. Moreover, multiple copies of the consensus motif both bind ElrB and support translational control. Therefore, there is a direct correlation between ElrB binding and translational repression by the Vg1 3'-UTR. In agreement with the reporter data, injection of a monoclonal antibody against ElrB into Xenopus oocytes resulted in the production of Vg1 protein, arguing for a role for the ELAV proteins in the translational repression of Vg1 mRNA during early oogenesis.
Figures




References
-
- Atasoy, U., J. Watson, D. Patel, and J. D. Keene. 1998. ELAV protein HuA (HuR) can redistribute between nucleus and cytoplasm and is upregulated during serum stimulation and T cell activation. J. Cell Sci. 111:3145-3156. - PubMed
-
- Campos, A. R., D. Grossman, and K. White. 1985. Mutant alleles at the locus elav in Drosophila melanogaster lead to nervous system defects. A developmental-genetic analysis. J. Neurogenet. 2:197-218. - PubMed
-
- Chen, C.-Y., and A.-B. Shyu. 1995. AU-rich elements: characterisation and importance in mRNA degradation. Trends Biochem. Sci. 20:465-470. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
