Pericardial heart disease: its morphologic features and its causes
- PMID: 16200146
- PMCID: PMC1200698
- DOI: 10.1080/08998280.2005.11928030
Pericardial heart disease: its morphologic features and its causes
Figures










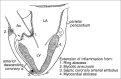







Similar articles
-
Pericardial heart disease: a study of its causes, consequences, and morphologic features.Cardiovasc Clin. 1976;7(3):11-65. Cardiovasc Clin. 1976. PMID: 1000531
-
Pericardial heart disease.Curr Probl Cardiol. 1977 Jun;2(3):1-71. doi: 10.1016/0146-2806(77)90006-8. Curr Probl Cardiol. 1977. PMID: 142612 Review.
-
Morphologic aspects of pericardial heart disease: Part I.Clin Cardiol. 1992 Mar;15(3):203-9. doi: 10.1002/clc.4960150312. Clin Cardiol. 1992. PMID: 1551268
-
Morphologic aspects of pericardial heart disease: Part II.Clin Cardiol. 1992 Apr;15(4):291-8. doi: 10.1002/clc.4960150413. Clin Cardiol. 1992. PMID: 1563133 Review.
-
Pericardial fluid from patients with ischemic heart disease induces myocardial cell apoptotis via an oxidant stress-sensitive p38 mitogen-activated protein kinase pathway.J Mol Cell Cardiol. 2001 Mar;33(3):419-30. doi: 10.1006/jmcc.2000.1314. J Mol Cell Cardiol. 2001. PMID: 11181011
Cited by
-
Spontaneous cardiac calcinosis in BALB/cByJ mice.Comp Med. 2013 Feb;63(1):29-37. Comp Med. 2013. PMID: 23561935 Free PMC article.
-
"Bread and butter" fibrinous pericarditis.Autops Case Rep. 2016 Dec 30;6(4):5-7. doi: 10.4322/acr.2016.050. eCollection 2016 Oct-Dec. Autops Case Rep. 2016. PMID: 28210567 Free PMC article. No abstract available.
-
Cardiac mesothelial papillary hyperplasia in four dogs.J Vet Diagn Invest. 2018 May;30(3):479-482. doi: 10.1177/1040638717753964. Epub 2018 Jan 11. J Vet Diagn Invest. 2018. PMID: 29322883 Free PMC article.
-
Cancer Treatment-Associated Pericardial Disease: Epidemiology, Clinical Presentation, Diagnosis, and Management.Curr Cardiol Rep. 2019 Nov 25;21(12):156. doi: 10.1007/s11886-019-1225-6. Curr Cardiol Rep. 2019. PMID: 31768769 Review.
-
External Compression of Epicardial Coronary Arteries with Partial Calcific Pericarditis.Heart Views. 2017 Jan-Mar;18(1):26-29. doi: 10.4103/1995-705X.206207. Heart Views. 2017. PMID: 28584590 Free PMC article.
References
-
- Osler W. Designed for the Use of Practitioners and Students of Medicine. New York: Appleton Co; 1892. The Principles and Practice of Medicine; p. 1079.
-
- Reeves RL. Cause of acute pericarditis. Am J Med Sci. 1953;225:34. - PubMed
-
- Griffith GC, Wallace L. The etiology of pericarditis. Am Heart J. 1949;37:636.
-
- Ishihara T, Ferrans VJ, Jones M, Boyce SW, Kawanami O, Roberts WC. Histologic and ultrastructural features of normal parietal pericardium. Am J Cardiol. 1980;46:744–753. - PubMed
-
- Shirani J, Berezowski K, Roberts WC. Quantitative measurement of normal and excessive (cor adiposum) subepicardial adipose tissue, its clinical significance, and its effect on electrocardiographic QRS voltage. Am J Cardiol. 1995;76:414–418. - PubMed
LinkOut - more resources
Full Text Sources
