Mitochondria: pharmacological manipulation of cell death
- PMID: 16200197
- PMCID: PMC1236694
- DOI: 10.1172/JCI26274
Mitochondria: pharmacological manipulation of cell death
Abstract
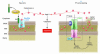
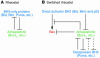
Cell death by apoptosis or necrosis is often important in the etiology and treatment of disease. Since mitochondria play important roles in cell death pathways, these organelles are potentially prime targets for therapeutic intervention. Here we discuss the mechanisms through which mitochondria participate in the cell death process and also survey some of the pharmacological approaches that target mitochondria in various ways.
Figures
References
-
- Weissig V, Boddapati SV, D’Souza GGM, Cheng SM. Targeting of low-molecular weight drugs to mammalian mitochondria. Drug Design Reviews – Online. 2004;1:15–28.
-
- Martin SJ, Green DR. Protease activation during apoptosis: death by a thousand cuts? Cell. 1995;82:349–352. - PubMed
-
- Newmeyer DD, Ferguson-Miller S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell. 2003;112:481–490. - PubMed
-
- Adrain C, Martin SJ. The mitochondrial apoptosome: a killer unleashed by the cytochrome seas. Trends Biochem. Sci. 2001;26:390–397. - PubMed
-
- Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. - PubMed

