Endoplasmic reticulum stress: cell life and death decisions
- PMID: 16200199
- PMCID: PMC1236697
- DOI: 10.1172/JCI26373
Endoplasmic reticulum stress: cell life and death decisions
Abstract
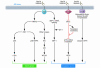
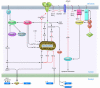
Disturbances in the normal functions of the ER lead to an evolutionarily conserved cell stress response, the unfolded protein response, which is aimed initially at compensating for damage but can eventually trigger cell death if ER dysfunction is severe or prolonged. The mechanisms by which ER stress leads to cell death remain enigmatic, with multiple potential participants described but little clarity about which specific death effectors dominate in particular cellular contexts. Important roles for ER-initiated cell death pathways have been recognized for several diseases, including hypoxia, ischemia/reperfusion injury, neurodegeneration, heart disease, and diabetes.
Figures
References
-
- Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat. Res. 2005;569:29–63. - PubMed
-
- Shen X, Zhang K, Kaufman RJ. The unfolded protein response: a stress signaling pathway of the endoplasmic reticulum [review] J. Chem. Neuroanat. 2004;28:79–92. - PubMed
-
- Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11:372–380. - PubMed
-
- Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003;22:8608–8618. - PubMed
-
- Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link [review] Nat. Rev. Mol. Cell Biol. 2003;4:552–565. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

