Deficits in amygdaloid cAMP-responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism
- PMID: 16200210
- PMCID: PMC1236671
- DOI: 10.1172/JCI24381
Deficits in amygdaloid cAMP-responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism
Erratum in
- J Clin Invest. 2006 Dec;116(12):3292
Abstract
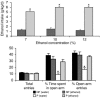
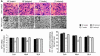
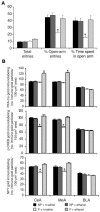
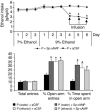
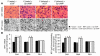
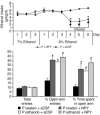
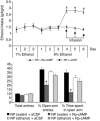
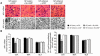
We investigated the role of cAMP-responsive element-binding protein (CREB) in genetic predisposition to anxiety and alcohol-drinking behaviors using alcohol-preferring (P) and -nonpreferring (NP) rats. The levels of CREB, phosphorylated CREB, and neuropeptide Y (NPY) were innately lower in the central amygdala (CeA) and medial amygdala (MeA), but not in the basolateral amygdala (BLA), of P rats compared with NP rats. P rats displayed higher baseline anxiety-like behaviors and consumed higher amounts of alcohol compared with NP rats. Ethanol injection or voluntary intake reduced the higher anxiety levels in P rats. Ethanol also increased CREB function in the CeA and MeA, but not in the BLA, of P rats. Infusion of the PKA activator Sp-cAMP or NPY into the CeA decreased the alcohol intake and anxiety-like behaviors of P rats. PKA activator infusion also increased CREB function in the CeA of P rats. On the other hand, ethanol injection or voluntary intake did not produce any changes either in anxiety levels or on CREB function in the amygdaloid structures of NP rats. Interestingly, infusion of the PKA inhibitor Rp-cAMP into the CeA provoked anxiety-like behaviors and increased alcohol intake in NP rats. PKA inhibitor decreased CREB function in the CeA of NP rats. These novel results provide the first evidence to our knowledge that decreased CREB function in the CeA may be operative in maintaining the high anxiety and excessive alcohol-drinking behaviors of P rats.
Figures




Comment in
-
The anxious amygdala: CREB signaling and predisposition to anxiety and alcoholism.J Clin Invest. 2005 Oct;115(10):2697-9. doi: 10.1172/JCI26436. J Clin Invest. 2005. PMID: 16200206 Free PMC article. Review.
Similar articles
-
The anxious amygdala: CREB signaling and predisposition to anxiety and alcoholism.J Clin Invest. 2005 Oct;115(10):2697-9. doi: 10.1172/JCI26436. J Clin Invest. 2005. PMID: 16200206 Free PMC article. Review.
-
The decreased cyclic-AMP dependent-protein kinase A function in the nucleus accumbens: a role in alcohol drinking but not in anxiety-like behaviors in rats.Neuropsychopharmacology. 2006 Jul;31(7):1406-19. doi: 10.1038/sj.npp.1300900. Epub 2005 Sep 28. Neuropsychopharmacology. 2006. PMID: 16192983
-
The decreased phosphorylation of cyclic adenosine monophosphate (cAMP) response element binding (CREB) protein in the central amygdala acts as a molecular substrate for anxiety related to ethanol withdrawal in rats.Alcohol Clin Exp Res. 2003 Mar;27(3):396-409. doi: 10.1097/01.ALC.0000056616.81971.49. Alcohol Clin Exp Res. 2003. PMID: 12658105
-
Neuropeptide Y signaling in the central nucleus of amygdala regulates alcohol-drinking and anxiety-like behaviors of alcohol-preferring rats.Alcohol Clin Exp Res. 2010 Mar 1;34(3):451-61. doi: 10.1111/j.1530-0277.2009.01109.x. Epub 2009 Dec 18. Alcohol Clin Exp Res. 2010. PMID: 20028368 Free PMC article.
-
Anxiety and alcohol abuse disorders: a common role for CREB and its target, the neuropeptide Y gene.Trends Pharmacol Sci. 2003 Sep;24(9):456-60. doi: 10.1016/S0165-6147(03)00226-8. Trends Pharmacol Sci. 2003. PMID: 12967770 Review.
Cited by
-
Behavioral Neuroadaptation to Alcohol: From Glucocorticoids to Histone Acetylation.Front Psychiatry. 2016 Oct 6;7:165. doi: 10.3389/fpsyt.2016.00165. eCollection 2016. Front Psychiatry. 2016. PMID: 27766083 Free PMC article. Review.
-
The influence of stress on the transition from drug use to addiction.Alcohol Res Health. 2008;31(2):119-36. Alcohol Res Health. 2008. PMID: 23584814 Free PMC article. Review.
-
CREB-mediated synaptogenesis and neurogenesis is crucial for the role of 5-HT1a receptors in modulating anxiety behaviors.Sci Rep. 2016 Jul 12;6:29551. doi: 10.1038/srep29551. Sci Rep. 2016. PMID: 27404655 Free PMC article.
-
Convergent functional genomics of anxiety disorders: translational identification of genes, biomarkers, pathways and mechanisms.Transl Psychiatry. 2011 May 24;1(5):e9. doi: 10.1038/tp.2011.9. Transl Psychiatry. 2011. PMID: 22832404 Free PMC article.
-
Alcohol disinhibition of behaviors in C. elegans.PLoS One. 2014 Mar 28;9(3):e92965. doi: 10.1371/journal.pone.0092965. eCollection 2014. PLoS One. 2014. PMID: 24681782 Free PMC article.
References
-
- National Institute on Alcohol Abuse and Alcoholism. 1993. Eighth special report to the congress on alcohol and health. US Department of Health and Human Services, NIH. Bethesda, Maryland, USA.
-
- Enoch MA. Pharmacogenomics of alcohol response and addiction. Am. J. Pharmacogenomics. 2003;3:217–232. - PubMed
-
- Radel M, Goldman D. Pharmacogenetics of alcohol response and alcoholism: the interplay of genes and environmental factors in thresholds for alcoholism. Drug Metab. Dispos. 2001;29:489–494. - PubMed
-
- Thome J, Gewirtz JC, Weijers HG, Wiesbeck GA, Henn FA. Genome polymorphism and alcoholism. Pharmacogenomics. 2000;1:63–71. - PubMed
-
- Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

