A molecular chaperone for mitochondrial complex I assembly is mutated in a progressive encephalopathy
- PMID: 16200211
- PMCID: PMC1236688
- DOI: 10.1172/JCI26020
A molecular chaperone for mitochondrial complex I assembly is mutated in a progressive encephalopathy
Abstract
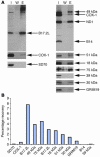
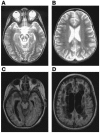
NADH:ubiquinone oxidoreductase (complex I) deficiency is a common cause of mitochondrial oxidative phosphorylation disease. It is associated with a wide range of clinical phenotypes in infants, including Leigh syndrome, cardiomyopathy, and encephalomyopathy. In at least half of patients, enzyme deficiency results from a failure to assemble the holoenzyme complex; however, the molecular chaperones required for assembly of the mammalian enzyme remain unknown. Using whole genome subtraction of yeasts with and without a complex I to generate candidate assembly factors, we identified a paralogue (B17.2L) of the B17.2 structural subunit. We found a null mutation in B17.2L in a patient with a progressive encephalopathy and showed that the associated complex I assembly defect could be completely rescued by retroviral expression of B17.2L in patient fibroblasts. An anti-B17.2L antibody did not associate with the holoenzyme complex but specifically recognized an 830-kDa subassembly in several patients with complex I assembly defects and coimmunoprecipitated a subset of complex I structural subunits from normal human heart mitochondria. These results demonstrate that B17.2L is a bona fide molecular chaperone that is essential for the assembly of complex I and for the normal function of the nervous system.
Figures


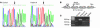


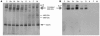
Comment in
-
Mining yeast in silico unearths a golden nugget for mitochondrial biology.J Clin Invest. 2005 Oct;115(10):2689-91. doi: 10.1172/JCI26625. J Clin Invest. 2005. PMID: 16200203 Free PMC article. Review.
References
-
- Friedrich T, Weiss H. Modular evolution of the respiratory NADH:ubiquinone oxidoreductase and the origin of its modules. J. Theor. Biol. 1997;187:529–540. - PubMed
-
- Abdrakhmanova A, et al. Subunit composition of mitochondrial complex I from the yeast Yarrowia lipolytica. . Biochim. Biophys. Acta. 2004; 1658:148–156. - PubMed
-
- Carroll J, Fearnley IM, Shannon RJ, Hirst J, Walker JE. Analysis of the subunit composition of complex I from bovine heart mitochondria. Mol. Cell. Proteomics. 2003;2:117–126. - PubMed
-
- Gabaldon T, Rainey D, Huynen MA. Tracing the evolution of a large protein complex in the eukaryotes, NADH:ubiquinone oxidoreductase (complex I) J. Mol. Biol. 2005;348:857–870. - PubMed
-
- Grigorieff N. Three-dimensional structure of bovine NADH:ubiquinone oxidoreductase (complex I) at 22 A in ice. J. Mol. Biol. 1998;277:1033–1046. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

