Changes in the expression of plasma membrane calcium extrusion systems during the maturation of hippocampal neurons
- PMID: 16200642
- PMCID: PMC3873839
- DOI: 10.1002/hipo.20129
Changes in the expression of plasma membrane calcium extrusion systems during the maturation of hippocampal neurons
Abstract
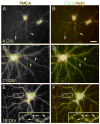
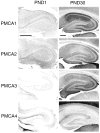
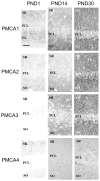
Spatial and temporal control of intracellular calcium signaling is essential for neuronal development and function. The termination of local Ca2+ signaling and the maintenance of basal Ca2+ levels require specific extrusion systems in the plasma membrane. In rat hippocampal neurons (HNs) developing in vitro, transcripts for all isoforms of the plasma membrane Ca2+ pump and the Na/Ca2+ exchanger, and the major nonphotoreceptor Na+/Ca2+,K+ exchangers (NCKX) were strongly upregulated during the second week in culture. Upregulation of plasma membrane calcium ATPases (PMCAs)1, 3, and 4 mRNA coincided with a splice shift from the ubiquitous b-type to the neuron-specific a-type with altered calmodulin regulation. Expression of all PMCA isoforms increased over 5-fold during the first 2 weeks. PMCA immunoreactivity was initially concentrated in the soma and growth cones of developing HNs. As the cells matured, PMCAs concentrated in the dendritic membrane and often colocalized with actin-rich dendritic spines in mature neurons. In the developing rat hippocampal CA1 region, immunohistochemistry confirmed the upregulation of all PMCAs and showed that by the end of the second postnatal week, PMCAs1, 2, and 3 were concentrated in the neuropil, with less intense staining of cell bodies in the pyramidal layer. PMCA4 staining was restricted to a few cells showing intense labeling of the cell periphery and neurites. These results establish that all major Ca2+ extrusion systems are strongly upregulated in HNs during the first 2 weeks of postnatal development. The overall increase in Ca2+ extrusion systems is accompanied by changes in the expression and cellular localization of different isoforms of the Ca2+ pumps and exchangers. The accumulation of PMCAs in dendrites and dendritic spines coincides with the functional maturation in these neurons, suggesting the importance of the proper spatial organization of Ca2+ extrusion systems for synaptic function and development.
Copyright 2005 Wiley-Liss, Inc.
Figures



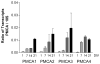

References
-
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. - PubMed
-
- Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. - PubMed
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York, NY: John Wiley and Sons; 1998.
-
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nature Rev Mol Cell Biol. 2003;4:517–529. - PubMed
-
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: Its physiological implications. Physiol Rev. 1999;79:763–854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

