Investigating bacterial N-linked glycosylation: synthesis and glycosyl acceptor activity of the undecaprenyl pyrophosphate-linked bacillosamine
- PMID: 16201778
- PMCID: PMC1351108
- DOI: 10.1021/ja054265v
Investigating bacterial N-linked glycosylation: synthesis and glycosyl acceptor activity of the undecaprenyl pyrophosphate-linked bacillosamine
Abstract
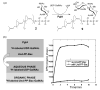
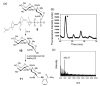
The chemical synthesis and biological activity of undecaprenyl pyrophosphate bacillosamine (Und-PP-Bac), an obligatory intermediate in the asparagine-linked glycosylation pathway of Campylobacter jejuni, are reported. The key transformation involves the coupling of bacillosamine phosphate and undecaprenyl phosphate. The synthetic Und-PP-Bac can be used to investigate the activity of the enzyme PglA, which catalyzes the first glycosyl transfer in substrate biosynthesis for N-linked protein glycosylation in the pathogenic gram-negative bacterium. The availability of this synthetic substrate makes it possible to access polyprenyl-linked oligosaccharides, such as the GalNAc-alpha-1,3-bacillosamine-alpha-1-PP-Und intermediate, that will enable exploration of the remaining enzymes in the prokaryotic glycosylation pathway. Study of the bacterial glycosylation system will provide insight into the corresponding eukaryotic process, which is currently poorly understood.
Figures
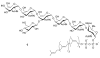
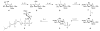
References
-
- Burda P, Aebi M. Biochim Biophys Acta. 1999;1426:239–257. - PubMed
-
- Nita-Lazar M, Wacker M, Schegg B, Amber S, Aebi M. Glycobiology. 2005;15:1957–1964. - PubMed
-
- Young MN, Brisson JR, Kelly J, Watson DC, Tessier L, Lanthier PH, Jarrell HC, Cadotte N, St Michael F, Aberg E, Szymanski CM. J Biol Chem. 2002;277:42530–42539. - PubMed
-
- Wacker M, Linton D, Hitchen PG, Nita-Lazar M, Haslam SM, North SJ, Panico M, Morris HR, Dell A, Wren BW, Aebi M. Science. 2002;298:1790–1793. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

