Combustion of dried animal dung as biofuel results in the generation of highly redox active fine particulates
- PMID: 16202154
- PMCID: PMC1262769
- DOI: 10.1186/1743-8977-2-6
Combustion of dried animal dung as biofuel results in the generation of highly redox active fine particulates
Abstract
Background: The burning of biomass in the developing world for heating and cooking results in high indoor particle concentrations. Long-term exposure to airborne particulate matter (PM) has been associated with increased rates of acute respiratory infections, chronic obstructive lung disease and cancer. In this study we determined the oxidative activity of combustion particles derived from the biomass fuel dung cake by examining their capacity to deplete antioxidants from a model human respiratory tract lining fluid (RTLF). For comparison, the observed oxidative activity was compared with that of particles derived from industrial and vehicular sources.
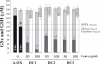
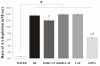
Results: Incubation of the dung cake particle suspensions in the RTLF for 4 h resulted in a mean loss of ascorbate of 72.1 +/- 0.7 and 89.7 +/- 2.5% at 50 and 100 microg/ml, respectively. Reduced glutathione was depleted by 49.6 +/- 4.3 and 63.5 +/- 22.4% under the same conditions. The capacity of these samples to deplete ascorbate was in excess of that observed with diesel or gasoline particles, but comparable to that seen with residual oil fly ash and considerably in excess of all three control particles in terms of glutathione depletion. Co-incubation with the metal chelator diethylenetriaminepentaacetate inhibited these losses, whilst minimal inhibition was seen with superoxide dismutase and catalase treatment. The majority of the activity observed appeared to be contained within aqueous particle extracts.
Conclusion: These data demonstrate that biomass derived particles have considerable oxidative activity, largely attributable to their transition metal content.
Figures


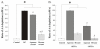
Similar articles
-
Oxidative potential of smoke from burning wood and mixed biomass fuels.Free Radic Res. 2013 Oct;47(10):829-35. doi: 10.3109/10715762.2013.832831. Free Radic Res. 2013. PMID: 23926954
-
Particle characteristics responsible for effects on human lung epithelial cells.Res Rep Health Eff Inst. 2002 Dec;(110):1-65; discussion 67-76. Res Rep Health Eff Inst. 2002. PMID: 12578113 Review.
-
Air pollution particles mediated oxidative DNA base damage in a cell free system and in human airway epithelial cells in relation to particulate metal content and bioreactivity.Chem Res Toxicol. 2001 Jul;14(7):879-87. doi: 10.1021/tx010022e. Chem Res Toxicol. 2001. PMID: 11453735
-
Chemical, microphysical and optical properties of primary particles from the combustion of biomass fuels.Environ Sci Technol. 2008 Dec 1;42(23):8829-34. doi: 10.1021/es800943f. Environ Sci Technol. 2008. PMID: 19192805
-
Air pollution combustion emissions: characterization of causative agents and mechanisms associated with cancer, reproductive, and cardiovascular effects.Mutat Res. 2007 Nov-Dec;636(1-3):95-133. doi: 10.1016/j.mrrev.2007.08.003. Epub 2007 Aug 17. Mutat Res. 2007. PMID: 17951105 Review.
Cited by
-
The effect of exposure to biomass smoke on respiratory symptoms in adult rural and urban Nepalese populations.Environ Health. 2014 Nov 6;13:92. doi: 10.1186/1476-069X-13-92. Environ Health. 2014. PMID: 25374400 Free PMC article.
-
Analysis of fine particulates from fuel burning in a reconstructed building at Çatalhöyük World Heritage Site, Turkey: assessing air pollution in prehistoric settled communities.Environ Geochem Health. 2022 Mar;44(3):1033-1048. doi: 10.1007/s10653-021-01000-2. Epub 2021 Jun 21. Environ Geochem Health. 2022. PMID: 34155558 Free PMC article.
-
Comparison of in vitro toxicological effects of biomass smoke from different sources of animal dung.Toxicol In Vitro. 2017 Sep;43:76-86. doi: 10.1016/j.tiv.2017.05.021. Epub 2017 May 30. Toxicol In Vitro. 2017. PMID: 28572013 Free PMC article.
-
A Systematic Review of Innate Immunomodulatory Effects of Household Air Pollution Secondary to the Burning of Biomass Fuels.Ann Glob Health. 2015 May-Jun;81(3):368-74. doi: 10.1016/j.aogh.2015.08.006. Ann Glob Health. 2015. PMID: 26615071 Free PMC article.
-
Use of Cooking Fuels and Cataract in a Population-Based Study: The India Eye Disease Study.Environ Health Perspect. 2016 Dec;124(12):1857-1862. doi: 10.1289/EHP193. Epub 2016 May 25. Environ Health Perspect. 2016. PMID: 27227523 Free PMC article.
References
-
- Barnes DF, Openshaw K, Smith KR, van der Plas R. What makes people cook with improved biomass stoves? A Comparative International Review of Stove Programs. World Bank Technical Paper. 1994;242 World Bank.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

