ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal
- PMID: 16202168
- PMCID: PMC1277830
- DOI: 10.1186/1475-2867-5-30
ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal
Abstract
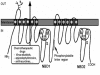
One of the major problems related with anticancer chemotherapy is resistance against anticancer drugs. The ATP-binding cassette (ABC) transporters are a family of transporter proteins that are responsible for drug resistance and a low bioavailability of drugs by pumping a variety of drugs out cells at the expense of ATP hydrolysis. One strategy for reversal of the resistance of tumor cells expressing ABC transporters is combined use of anticancer drugs with chemosensitizers. In this review, the physiological functions and structures of ABC transporters, and the development of chemosensitizers are described focusing on well-known proteins including P-glycoprotein, multidrug resistance associated protein, and breast cancer resistance protein.
Figures
References
-
- Stavrovskaya AA. Cellular mechanisms of multidrug resistance of tumor cells. Biochemistry (Mosc) 2000;65:95–106. - PubMed
-
- Riordan JR, Ling V. Purification of P-glycoprotein from plasma membrane vesicles of Chinese hamster ovary cell mutants with reduced colchicine permeability. J Biol Chem. 1979;254:12701–12705. - PubMed
-
- Di Pietro A, Dayan G, Conseil G, Steinfels E, Krell T, Trompier D, Baubichon-Cortay H, Jault J. P-glycoprotein-mediated resistance to chemotherapy in cancer cells: using recombinant cytosolic domains to establish structure-function relationships. Braz J Med Biol Res. 1999;32:925–939. doi: 10.1590/S0100-879X1999000800001. - DOI - PubMed
-
- Dean M, Hamon Y, Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res. 2001;42:1007–1017. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

