Enzymatically active ADAMTS13 variants are not inhibited by anti-ADAMTS13 autoantibodies: a novel therapeutic strategy?
- PMID: 16203734
- PMCID: PMC2582217
- DOI: 10.1074/jbc.M504919200
Enzymatically active ADAMTS13 variants are not inhibited by anti-ADAMTS13 autoantibodies: a novel therapeutic strategy?
Abstract
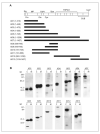
ADAMTS13 (a disintegrin and metalloprotease with thrombospondin motifs), a circulating multidomain zinc metalloprotease of the reprolysin subfamily, is critical for preventing von Willebrand factor-platelet interaction under high shear stress conditions. A deficiency of the protease, due to mutations in the ADAMTS13 gene or the presence of antibodies that inhibit the activity of the protease, causes thrombotic thrombocytopenic purpura (TTP). Plasma therapy, the conventional therapy for TTP, may cause serious adverse reactions and is ineffective in some patients. In order to develop new strategies for improving the diagnosis and treatment of TTP, we produced a series of truncated ADAMTS13 proteins in mammalian cells and analyzed their binding with and suppression by the IgG derived from the TTP patients. The results revealed that truncation of the ADAMTS13 protein at its cysteine-rich region eliminated its recognition by the antibodies without abolishing its von Willebrand factor-cleaving activity. This raises the possibility that resistant ADAMTS13 variants may be exploited to circumvent inhibitory antibodies that cause TTP.
Figures





References
-
- Handin RI, Wagner DD. Prog. Hemost. Thromb. 1989;9:233–259. - PubMed
-
- Tsai HM. Semin. Thromb. Hemost. 2003;29:479–488. - PubMed
-
- Tsai HM. Semin. Thromb. Hemost. 2004;30:549–557. - PubMed
-
- Bukowski RM. Prog. Hemost. Thromb. 1982;6:287–337. - PubMed
-
- Furlan M, Robles R, Solenthaler M, Lammle B. Blood. 1998;91:2839–2846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

