Rootletin forms centriole-associated filaments and functions in centrosome cohesion
- PMID: 16203858
- PMCID: PMC2171225
- DOI: 10.1083/jcb.200504107
Rootletin forms centriole-associated filaments and functions in centrosome cohesion
Abstract
After duplication of the centriole pair during S phase, the centrosome functions as a single microtubule-organizing center until the onset of mitosis, when the duplicated centrosomes separate for bipolar spindle formation. The mechanisms regulating centrosome cohesion and separation during the cell cycle are not well understood. In this study, we analyze the protein rootletin as a candidate centrosome linker component. As shown by immunoelectron microscopy, endogenous rootletin forms striking fibers emanating from the proximal ends of centrioles. Moreover, rootletin interacts with C-Nap1, a protein previously implicated in centrosome cohesion. Similar to C-Nap1, rootletin is phosphorylated by Nek2 kinase and is displaced from centrosomes at the onset of mitosis. Whereas the overexpression of rootletin results in the formation of extensive fibers, small interfering RNA-mediated depletion of either rootletin or C-Nap1 causes centrosome splitting, suggesting that both proteins contribute to maintaining centrosome cohesion. The ability of rootletin to form centriole-associated fibers suggests a dynamic model for centrosome cohesion based on entangling filaments rather than continuous polymeric linkers.
Figures
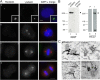

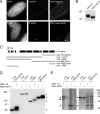
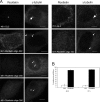
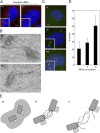
References
-
- Andersen, J.S., C.J. Wilkinson, T. Mayor, P. Mortensen, E.A. Nigg, and M. Mann. 2003. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 426:570–574. - PubMed
-
- Bornens, M. 2002. Centrosome composition and microtubule anchoring mechanisms. Curr. Opin. Cell Biol. 14:25–34. - PubMed
-
- Bornens, M., M. Paintrand, J. Berges, M.C. Marty, and E. Karsenti. 1987. Structural and chemical characterization of isolated centrosomes. Cell Motil. Cytoskeleton. 8:238–249. - PubMed
-
- Elbashir, S.M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 411:494–498. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

