The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis
- PMID: 16203868
- PMCID: PMC2213176
- DOI: 10.1084/jem.20051239
The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis
Abstract
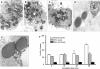
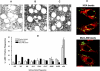
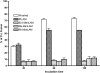
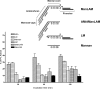
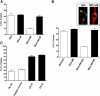
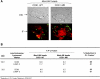
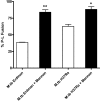
Mycobacterium tuberculosis (M.tb) survives in macrophages in part by limiting phagosome-lysosome (P-L) fusion. M.tb mannose-capped lipoarabinomannan (ManLAM) blocks phagosome maturation. The pattern recognition mannose receptor (MR) binds to the ManLAM mannose caps and mediates phagocytosis of bacilli by human macrophages. Using quantitative electron and confocal microscopy, we report that engagement of the MR by ManLAM during the phagocytic process is a key step in limiting P-L fusion. P-L fusion of ManLAM microspheres was significantly reduced in human macrophages and an MR-expressing cell line but not in monocytes that lack the receptor. Moreover, reversal of P-L fusion inhibition occurred with MR blockade. Inhibition of P-L fusion did not occur with entry via Fcgamma receptors or dendritic cell-specific intracellular adhesion molecule 3 grabbing nonintegrin, or with phosphatidylinositol-capped lipoarabinomannan. The ManLAM mannose cap structures were necessary in limiting P-L fusion, and the intact molecule was required to maintain this phenotype. Finally, MR blockade during phagocytosis of virulent M.tb led to a reversal of P-L fusion inhibition in human macrophages (84.0 +/- 5.1% vs. 38.6 +/- 0.6%). Thus, engagement of the MR by ManLAM during the phagocytic process directs M.tb to its initial phagosomal niche, thereby enhancing survival in human macrophages.
Figures


References
-
- Fenton, M.J., L.W. Riley, and L.S. Schlesinger. 2005. Receptor-mediated recognition of Mycobacterium tuberculosis by host cells. Tuberculosis and the Tubercle Bacillus. S.T. Cole, K.D. Eisenach, D.N. McMurray, and W.R. Jacobs, Jr., editors. ASM Press, New York. 405–426.
-
- Brennan, P.J., and H. Nikaido. 1995. The envelope of mycobacteria. Annu. Rev. Biochem. 64:29–63. - PubMed
-
- Strohmeier, G.R., and M.J. Fenton. 1999. Roles of lipoarabinomannan in the pathogenesis of tuberculosis. Microbes Infect. 1:709–717. - PubMed
-
- Hunter, S.W., and P.J. Brennan. 1990. Evidence for the presence of a phosphatidylinositol anchor on the lipoarabinomannan and lipomannan of Mycobacterium tuberculosis. J. Biol. Chem. 265:9272–9279. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

