Altered right atrial excitation and propagation in connexin40 knockout mice
- PMID: 16203917
- PMCID: PMC2956435
- DOI: 10.1161/CIRCULATIONAHA.104.527325
Altered right atrial excitation and propagation in connexin40 knockout mice
Abstract
Background: Intercellular coupling via connexin40 (Cx40) gap junction channels is an important determinant of impulse propagation in the atria.
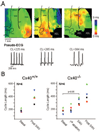
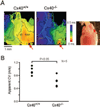
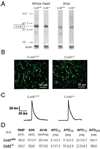
Methods and results: We studied the role of Cx40 in intra-atrial excitation and propagation in wild-type (Cx40(+/+)) and knockout (Cx40(-/-)) mice using high-resolution, dual-wavelength optical mapping. On ECG, the P wave was significantly prolonged in Cx40(-/-) mice (13.4+/-0.5 versus 11.4+/-0.3 ms in Cx40(+/+)). In Cx40(+/+) hearts, spontaneous right atrial (RA) activation showed a focal breakthrough at the junction of the right superior vena cava, sulcus terminalis, and RA free wall, corresponding to the location of the sinoatrial node. In contrast, Cx40(-/-) hearts displayed ectopic breakthrough sites at the base of the sulcus terminalis, RA free wall, and right superior vena cava. Progressive ablation of such sites in 4 Cx40(-/-) mice resulted in ectopic focus migration and cycle length prolongation. In all Cx40(-/-) hearts the focus ultimately shifted to the sinoatrial node at a very prolonged cycle length (initial ectopic cycle length, 182+/-20 ms; postablation sinus cycle length, 387+/-44 ms). In a second group of experiments, epicardial pacing at 10 Hz revealed slower conduction in the RA free wall of 5 Cx40(-/-) hearts than in 5 Cx40(+/+) hearts (0.61+/-0.07 versus 0.94+/-0.07 m/s; P<0.05). Dominant frequency analysis in Cx40(-/-) RA demonstrated significant reduction in the area of 1:1 conduction at 16 Hz (40+/-10% versus 69+/-5% in Cx40(+/+)) and 25 Hz (36+/-11% versus 65+/-9% in Cx40(+/+)).
Conclusions: This is the first demonstration of intra-atrial block, ectopic rhythms, and altered atrial propagation in the RA of Cx40(-/-) mice.
Figures





References
-
- Kleber AGFV, Rohr S. Continuous and discontinuous propagation. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside. Philadelphia, Pa: WB Saunders Co; 2000. pp. 205–213.
-
- Jongsma HJ, Wilders R. Gap junctions in cardiovascular disease. Circ Res. 2000;86:1193–1197. - PubMed
-
- Firouzi M, Ramanna H, Kok B, Jongsma HJ, Koeleman BP, Doevendans PA, Groenewegen WA, Hauer RN. Association of human connexin40 gene polymorphisms with atrial vulnerability as a risk factor for idiopathic atrial fibrillation. Circ Res. 2004;95:e29–e33. - PubMed
-
- Groenewegen WA, Firouzi M, Bezzina CR, Vliex S, van Langen IM, Sandkuijl L, Smits JP, Hulsbeek M, Rook MB, Jongsma HJ, Wilde AA. A cardiac sodium channel mutation cosegregates with a rare connexin40 genotype in familial atrial standstill. Circ Res. 2003;92:14–22. - PubMed
-
- Gros D, Jarry-Guichard T, Ten Velde I, de Maziere A, van Kempen MJ, Davoust J, Briand JP, Moorman AF, Jongsma HJ. Restricted distribution of connexin40, a gap junctional protein, in mammalian heart. Circ Res. 1994;74:839–851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

