Inhibition of hypothalamic fatty acid synthase triggers rapid activation of fatty acid oxidation in skeletal muscle
- PMID: 16203972
- PMCID: PMC1253598
- DOI: 10.1073/pnas.0507300102
Inhibition of hypothalamic fatty acid synthase triggers rapid activation of fatty acid oxidation in skeletal muscle
Abstract
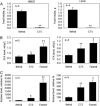
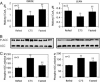
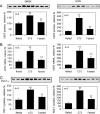
Malonyl-CoA functions as a mediator in the hypothalamic sensing of energy balance and regulates the neural physiology that governs feeding behavior and energy expenditure. The central administration of C75, a potent inhibitor of the fatty acid synthase (FAS), increases malonyl-CoA concentration in the hypothalamus and suppresses food intake while activating fatty acid oxidation in skeletal muscle. Closely correlated with the increase in muscle fatty acid oxidation is the phosphorylation/inactivation of acetyl-CoA carboxylase, which leads to reduced malonyl-CoA concentration. Lowering muscle malonyl-CoA, a potent inhibitor of carnitine/palmitoyl-CoA transferase 1 (CPT1), releases CPT1 from inhibitory constraint, facilitating the entry of fatty acids into mitochondria for beta oxidation. Also correlated with these events are C75-induced increases in the expression of skeletal muscle peroxisome proliferator-activated receptor alpha (PPARalpha), a transcriptional activator of fatty acid oxidizing enzymes, and uncoupling protein 3 (UCP3), a thermogenic mitochondrial uncoupling protein. Phentolamine, an alpha-adrenergic blocking agent, prevents the C75-induced increases of skeletal muscle UCP3 and whole body fatty acid oxidation and C75-induced decrease of skeletal muscle malonyl-CoA. Thus, the sympathetic nervous system is implicated in the transmission of the "malonyl-CoA signal" from brain to skeletal muscle. Consistent with the up-regulation of UCP3 and PPARalpha is the concomitant increase in the expression of PGC1alpha, transcriptional coactivator of the UCP3 and PPARalpha-activated genes. These findings clarify the mechanism by which the hypothalamic malonyl-CoA signal is communicated to metabolic systems in skeletal muscle that regulate fatty acid oxidation and energy expenditure.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

