Cdc7-Drf1 is a developmentally regulated protein kinase required for the initiation of vertebrate DNA replication
- PMID: 16204181
- PMCID: PMC1240038
- DOI: 10.1101/gad.1339805
Cdc7-Drf1 is a developmentally regulated protein kinase required for the initiation of vertebrate DNA replication
Abstract
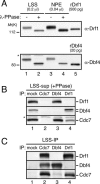
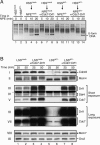
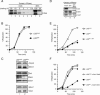
Cdc7, a protein kinase required for the initiation of eukaryotic DNA replication, is activated by a regulatory subunit, Dbf4. A second activator of Cdc7 called Drf1 exists in vertebrates, but its function is unknown. Here, we report that in Xenopus egg extracts, Cdc7-Drf1 is far more abundant than Cdc7-Dbf4, and removal of Drf1 but not Dbf4 severely inhibits phosphorylation of Mcm4 and DNA replication. After gastrulation, when the cell cycle acquires somatic characteristics, Drf1 levels decline sharply and Cdc7-Dbf4 becomes the more abundant kinase. These results identify Drf1 as a developmentally regulated, essential activator of Cdc7 in Xenopus.
Figures
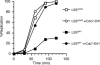

Similar articles
-
Xenopus CDC7/DRF1 complex is required for the initiation of DNA replication.J Biol Chem. 2006 Apr 28;281(17):11569-76. doi: 10.1074/jbc.M510278200. Epub 2006 Feb 28. J Biol Chem. 2006. PMID: 16507577
-
Drf1-dependent kinase interacts with Claspin through a conserved protein motif.J Biol Chem. 2010 Apr 23;285(17):12638-46. doi: 10.1074/jbc.M109.077370. Epub 2010 Feb 27. J Biol Chem. 2010. PMID: 20190277 Free PMC article.
-
DBF4, not DRF1, is the crucial regulator of CDC7 kinase at replication forks.J Cell Biol. 2024 Aug 5;223(8):e202402144. doi: 10.1083/jcb.202402144. Epub 2024 Jun 12. J Cell Biol. 2024. PMID: 38865090 Free PMC article.
-
Cdc7 kinase complex: a key regulator in the initiation of DNA replication.J Cell Physiol. 2002 Mar;190(3):287-96. doi: 10.1002/jcp.10070. J Cell Physiol. 2002. PMID: 11857444 Review.
-
Regulation and roles of Cdc7 kinase under replication stress.Cell Cycle. 2014;13(12):1859-66. doi: 10.4161/cc.29251. Epub 2014 May 19. Cell Cycle. 2014. PMID: 24841992 Free PMC article. Review.
Cited by
-
Evolutionary diversification of MCM3 genes in Xenopus laevis and Danio rerio.Cell Cycle. 2014;13(20):3271-81. doi: 10.4161/15384101.2014.954445. Cell Cycle. 2014. PMID: 25485507 Free PMC article.
-
Integrating S-phase checkpoint signaling with trans-lesion synthesis of bulky DNA adducts.Cell Biochem Biophys. 2007;47(3):392-408. doi: 10.1007/s12013-007-0032-7. Cell Biochem Biophys. 2007. PMID: 17652783 Free PMC article. Review.
-
Biphasic chromatin binding of histone chaperone FACT during eukaryotic chromatin DNA replication.Biochim Biophys Acta. 2011 Jun;1813(6):1129-36. doi: 10.1016/j.bbamcr.2011.01.002. Epub 2011 Jan 11. Biochim Biophys Acta. 2011. PMID: 21232560 Free PMC article.
-
Chk1 Inhibition of the Replication Factor Drf1 Guarantees Cell-Cycle Elongation at the Xenopus laevis Mid-blastula Transition.Dev Cell. 2017 Jul 10;42(1):82-96.e3. doi: 10.1016/j.devcel.2017.06.010. Dev Cell. 2017. PMID: 28697335 Free PMC article.
-
The MCM2-7 Complex: Roles beyond DNA Unwinding.Biology (Basel). 2024 Apr 13;13(4):258. doi: 10.3390/biology13040258. Biology (Basel). 2024. PMID: 38666870 Free PMC article. Review.
References
-
- Bell S.P. and Dutta, A. 2002. DNA replication in eukaryotic cells. Annu. Rev. Biochem. 71: 333-374. - PubMed
-
- Blow J.J. and Laskey, R.A. 1986. Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell 47: 577-587. - PubMed
-
- Brown G.W. and Kelly, T.J. 1998. Purification of Hsk1, a minichromosome maintenance protein kinase from fission yeast. J. Biol. Chem. 273: 22083-22090. - PubMed
-
- Costanzo V., Shechter, D., Lupardus, P.J., Cimprich, K.A., Gottesman, M., and Gautier, J. 2003. An ATR- and Cdc7-dependent DNA damage checkpoint that inhibits initiation of DNA replication. Mol. Cell 11: 203-213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
