Identification and analysis of Escherichia coli ribonuclease E dominant-negative mutants
- PMID: 16204212
- PMCID: PMC1456196
- DOI: 10.1534/genetics.105.048553
Identification and analysis of Escherichia coli ribonuclease E dominant-negative mutants
Abstract
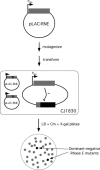
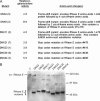
The Escherichia coli (E. coli) ribonuclease E protein (RNase E) is implicated in the degradation and processing of a large fraction of RNAs in the cell. To understand RNase E function in greater detail, we developed an efficient selection method for identifying nonfunctional RNase E mutants. A subset of the mutants was found to display a dominant-negative phenotype, interfering with wild-type RNase E function. Unexpectedly, each of these mutants contained a large truncation within the carboxy terminus of RNase E. In contrast, no point mutants that conferred a dominant-negative phenotype were found. We show that a representative dominant-negative mutant can form mixed multimers with RNase E and propose a model to explain how these mutants can block wild-type RNase E function in vivo.
Figures
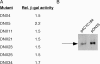



References
-
- Bolivar, F., 1978. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene 4: 121–136. - PubMed
-
- Bouvet, P., and J. G. Belasco, 1992. Control of RNase E-mediated RNA degradation by 5′-terminal base pairing in E. coli. Nature 360: 488–491. - PubMed
-
- Bycroft, M., T. J. Hubbard, M. Proctor, S. M. Freund and A. G. Murzin, 1997. The solution structure of the S1 RNA binding domain: a member of an ancient nucleic acid-binding fold. Cell 88: 235–242. - PubMed
-
- Callaghan, A. J., J. G. Grossmann, Y. U. Redko, L. L. Ilag, M. C. Moncrieffe et al., 2003. Quaternary structure and catalytic activity of the Escherichia coli ribonuclease E amino-terminal catalytic domain. Biochemistry 42: 13848–13855. - PubMed
-
- Carpousis, A. J., G. Van Houwe, C. Ehretsmann and H. M. Krisch, 1994. Co-purification of E. coli RNase E and PNPase: evidence for a specific association between two enzymes important in RNA processing and degradation. Cell 76: 889–900. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

