An aberrant sequence in a connexin46 mutant underlies congenital cataracts
- PMID: 16204255
- PMCID: PMC2720622
- DOI: 10.1074/jbc.M504765200
An aberrant sequence in a connexin46 mutant underlies congenital cataracts
Abstract
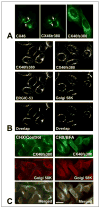
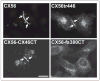
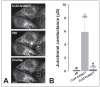
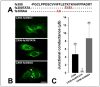
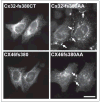
An increasing number of diseases have been mapped to genes coding for ion channel proteins, including the gap junction proteins, connexins. Here, we report on the identification of an amino acid sequence underlying the behavior of a non-functional mutant connexin46 (CX46) associated with congenital cataracts. The mutant protein, CX46fs380, is 31 amino acids longer than CX46 and contains 87 aberrant amino acids in its C terminus. When expressed in mammalian cells, the mutant CX46 was not found at gap junctional plaques, but it showed extensive co-localization with markers for ERGIC and Golgi. The severe reductions in function and formation of gap junctional plaques were transferred to other connexins by creating chimeras containing the last third (or more) of the aberrant C terminus of the CX46 mutant. This sequence also impaired trafficking of a CD8 chimera. Site-directed mutagenesis of a diphenylalanine restored appositional membrane localization and function. These results suggest a novel mechanism in which a mutation causes disease by generating a motif that leads to retention within the synthetic/secretory pathway.
Figures
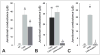


References
-
- Sáez JC, Berthoud VM, Branes MC, Martínez AD, Beyer EC. Physiol Rev. 2003;83:1359–1400. - PubMed
-
- Hübner CA, Jentsch TJ. Hum Mol Genet. 2002;11:2435–2445. - PubMed
-
- Reddy MA, Francis PJ, Berry V, Bhattacharya SS, Moore AT. Survey Ophthalmol. 2004;49:300–315. - PubMed
-
- Pal JD, Liu X, Mackay D, Shiels A, Berthoud VM, Beyer EC, Ebihara L. Am J Physiol Cell Physiol. 2000;279:C596–C602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

