Hematopoietic stem cells do not engraft with absolute efficiencies
- PMID: 16204316
- PMCID: PMC1895609
- DOI: 10.1182/blood-2005-02-0655
Hematopoietic stem cells do not engraft with absolute efficiencies
Abstract
Hematopoietic stem cells (HSCs) can be isolated from murine bone marrow by their ability to efflux the Hoechst 33342 dye. This method defines an extremely small and hematopoietically potent subset of cells known as the side population (SP). Recent studies suggest that transplanted single SP cells are capable of lymphohematopoietic repopulation at near absolute efficiencies. Here, we carefully reevaluate the hematopoietic potential of individual SP cells and find substantially lower rates of reconstitution. Our strategy involved the cotransplantation of single SP cells along with different populations of competitor cells that varied in their self-renewal capacity. Even with minimized HSC competition, SP cells were only able to reconstitute up to 35% of recipient mice. Furthermore, through immunophenotyping and clonal in vitro assays we find that SP cells are virtually homogeneous. Isolation of HSCs on the basis of Hoechst exclusion and a single cell-surface marker allows enrichment levels similar to that obtained with complex multicolor strategies. Altogether, our results indicate that even an extremely homogeneous HSC population, based on phenotype and dye efflux, cannot reconstitute mice at absolute efficiencies.
Figures
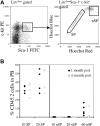


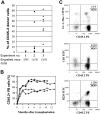
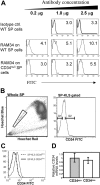
Comment in
-
The Hoechst low-fluorescent profile of the side population: clonogenicity versus dye retention.Blood. 2006 Sep 1;108(5):1774; author reply 1774-5. doi: 10.1182/blood-2006-03-013615. Blood. 2006. PMID: 16926296 No abstract available.
References
-
- Jordan CT, Lemischka IR. Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev. 1990;4: 220-232. - PubMed
-
- Lemischka IR, Raulet DH, Mulligan RC. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986;45: 917-927. - PubMed
-
- Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273: 242-245. - PubMed
-
- Morrison S, Weissman I. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1: 661-673. - PubMed
-
- Okada S, Nakauchi H, Nagayoshi K, Nishikawa S, Miura Y, Suda T. In vivo and in vitro stem cell function of c-kit- and Sca-1-positive murine hematopoietic cells. Blood. 1992;80: 3044-3050. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

