Genetic variation of the bloom-forming Cyanobacterium Microcystis aeruginosa within and among lakes: implications for harmful algal blooms
- PMID: 16204530
- PMCID: PMC1265933
- DOI: 10.1128/AEM.71.10.6126-6133.2005
Genetic variation of the bloom-forming Cyanobacterium Microcystis aeruginosa within and among lakes: implications for harmful algal blooms
Abstract
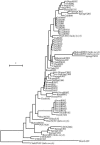
To measure genetic variation within and among populations of the bloom-forming cyanobacterium Microcystis aeruginosa, we surveyed a suite of lakes in the southern peninsula of Michigan that vary in productivity (total phosphorus concentrations of approximately 10 to 100 microg liter(-1)). Survival of M. aeruginosa isolates from lakes was relatively low (i.e., mean of 7% and maximum of 30%) and positively related to lake total phosphorus concentration (P = 0.014, r2 = 0.407, n = 14). In another study (D. F. Raikow, O. Sarnelle, A. E. Wilson, and S. K. Hamilton, Limnol. Oceanogr. 49:482-487, 2004), survival rates of M. aeruginosa isolates collected from an oligotrophic lake (total phosphorus of approximately 10 mug liter(-1) and dissolved inorganic nitrogen:total phosphorus ratio of 12.75) differed among five different medium types (G test, P of <0.001), with higher survival (P = 0.003) in low-nutrient media (28 to 37% survival) than in high-nutrient media. Even with the relatively low isolate survivorship that could select against detecting the full range of genetic variation, populations of M. aeruginosa were genetically diverse within and among lakes (by analysis of molecular variance, Phi(sc) = 0.412 [Phi(sc) is an F-statistic derivative which evaluates the correlation of haplotypic diversity within populations relative to the haplotypic diversity among all sampled populations], P = 0.001), with most clones being distantly related to clones collected from lakes directly attached to Lake Michigan (a Laurentian Great Lake) and culture collection strains collected from Canada, Scotland, and South Africa. Ninety-one percent of the 53 genetically unique M. aeruginosa clones contained the microcystin toxin gene (mcyA). Genotypes with the toxin gene were found in all lakes, while four lakes harbored both genotypes possessing and genotypes lacking the toxin gene.
Figures


References
-
- Barker, G. L. A., A. Konopka, B. A. Handley, and P. K. Hayes. 2000. Genetic variation in Aphanizomenon (cyanobacteria) colonies from the Baltic Sea and North America. J. Phycol. 36:947-950.
-
- Bittencourt-Oliveira, M. D., M. C. de Oliveira, and C. J. S. Bolch. 2001. Genetic variability of Brazilian strains of the Microcystis aeruginosa complex (Cyanobacteria/Cyanophyceae) using the phycocyanin intergenic spacer and flanking regions (cpcBA). J. Phycol. 37:810-818.
-
- Bolch, C. J. S., S. I. Blackburn, B. A. Neilan, and P. M. Grewe. 1996. Genetic characterization of strains of cyanobacteria using pcr-rflp of the cpcBA intergenic spacer and flanking regions. J. Phycol. 32:445-451.
-
- Bolch, C. J. S., P. T. Orr, G. J. Jones, and S. I. Blackburn. 1999. Genetic, morphological, and toxicological variation among globally distributed strains of Nodularia (cyanobacteria). J. Phycol. 35:339-355.
-
- Brand, L. E. 1988. Review of genetic variation in marine phytoplankton species and the ecological implications. Biol. Oceanogr. 6:397-409.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

