Effect of connective tissue growth factor (CCN2/CTGF) on proliferation and differentiation of mouse periodontal ligament-derived cells
- PMID: 16207372
- PMCID: PMC1276803
- DOI: 10.1186/1478-811X-3-11
Effect of connective tissue growth factor (CCN2/CTGF) on proliferation and differentiation of mouse periodontal ligament-derived cells
Abstract
Background: CCN2/CTGF is known to be involved in tooth germ development and periodontal tissue remodeling, as well as in mesenchymal tissue development and regeneration. In this present study, we investigated the roles of CCN2/CTGF in the proliferation and differentiation of periodontal ligament cells (murine periodontal ligament-derived cell line: MPL) in vitro.
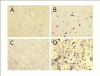
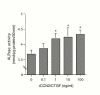
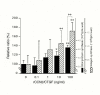
Results: In cell cultures of MPL, the mRNA expression of the CCN2/CTGF gene was stronger in sparse cultures than in confluent ones and was significantly enhanced by TGF-beta. The addition of recombinant CCN2/CTGF (rCCN2) to MPL cultures stimulated DNA synthesis and cell growth in a dose-dependent manner. Moreover, rCCN2 addition also enhanced the mRNA expression of alkaline phosphatase (ALPase), type I collagen, and periostin, the latter of which is considered to be a specific marker of the periosteum and periodontium; whereas it showed little effect on the mRNA expression of typical osteoblastic markers, e.g., osteopontin and osteocalcin. Finally, rCCN2/CTGF also stimulated ALPase activity and collagen synthesis.
Conclusion: These results taken together suggest important roles of CCN2/CTGF in the development and regeneration of periodontal tissue including the periodontal ligament.
Figures

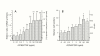

Similar articles
-
Effect of cellular communication network factor 2/connective tissue growth factor on tube formation by endothelial cells derived from human periodontal ligaments.Arch Oral Biol. 2021 Dec;132:105279. doi: 10.1016/j.archoralbio.2021.105279. Epub 2021 Oct 4. Arch Oral Biol. 2021. PMID: 34628139
-
Effect of transforming growth factor-beta1 on expression of the connective tissue growth factor (CCN2/CTGF) gene in normal human gingival fibroblasts and periodontal ligament cells.J Periodontal Res. 2009 Apr;44(2):161-9. doi: 10.1111/j.1600-0765.2008.01093.x. Epub 2008 Feb 6. J Periodontal Res. 2009. PMID: 19210343
-
Effects of enamel matrix derivative and transforming growth factor-β1 on connective tissue growth factor in human periodontal ligament fibroblasts.J Periodontol. 2015 Apr;86(4):569-77. doi: 10.1902/jop.2015.120448. Epub 2015 Jan 16. J Periodontol. 2015. PMID: 25594423
-
Regulation of hepatic stellate cells by connective tissue growth factor.Front Biosci (Landmark Ed). 2012 Jun 1;17(7):2495-507. doi: 10.2741/4067. Front Biosci (Landmark Ed). 2012. PMID: 22652794 Review.
-
CTGF/CCN2 from Skeletal Muscle to Nervous System: Impact on Neurodegenerative Diseases.Mol Neurobiol. 2019 Aug;56(8):5911-5916. doi: 10.1007/s12035-019-1490-9. Epub 2019 Jan 28. Mol Neurobiol. 2019. PMID: 30689195 Review.
Cited by
-
Gradient boosting-based classification of interactome hub genes in periimplantitis with periodontitis - an integrated bioinformatic approach.Front Oral Health. 2024 Nov 26;5:1462845. doi: 10.3389/froh.2024.1462845. eCollection 2024. Front Oral Health. 2024. PMID: 39659491 Free PMC article.
-
Inflammation-driven periostin in ECRS has contrasting effects on tissue structural integrity and osteitis.Front Immunol. 2025 Jun 18;16:1596746. doi: 10.3389/fimmu.2025.1596746. eCollection 2025. Front Immunol. 2025. PMID: 40607434 Free PMC article.
-
CCN2: a master regulator of the genesis of bone and cartilage.J Cell Commun Signal. 2013 Aug;7(3):191-201. doi: 10.1007/s12079-013-0204-8. J Cell Commun Signal. 2013. PMID: 23794334 Free PMC article.
-
Periostin as a novel biomarker for postoperative recurrence of chronic rhinosinitis with nasal polyps.Sci Rep. 2018 Jul 30;8(1):11450. doi: 10.1038/s41598-018-29612-2. Sci Rep. 2018. PMID: 30061580 Free PMC article.
-
CTGF Promotes the Osteoblast Differentiation of Human Periodontal Ligament Stem Cells by Positively Regulating BMP2/Smad Signal Transduction.Biomed Res Int. 2022 Sep 15;2022:2938015. doi: 10.1155/2022/2938015. eCollection 2022. Biomed Res Int. 2022. PMID: 36158888 Free PMC article.
References
-
- Bradham DM, Igarashi A, Potter RL, Grotendorst GR. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991;114:1285–1294. doi: 10.1083/jcb.114.6.1285. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

