BAG-2 acts as an inhibitor of the chaperone-associated ubiquitin ligase CHIP
- PMID: 16207813
- PMCID: PMC1289430
- DOI: 10.1091/mbc.e05-07-0660
BAG-2 acts as an inhibitor of the chaperone-associated ubiquitin ligase CHIP
Abstract
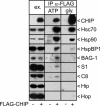
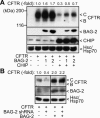
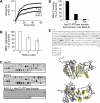
Cellular protein quality control involves a close interplay between molecular chaperones and the ubiquitin/proteasome system. We recently identified a degradation pathway, on which the chaperone Hsc70 delivers chaperone clients, such as misfolded forms of the cystic fibrosis transmembrane conductance regulator (CFTR), to the proteasome. The cochaperone CHIP is of central importance on this pathway, because it acts as a chaperone-associated ubiquitin ligase. CHIP mediates the attachment of a ubiquitin chain to a chaperone-presented client protein and thereby stimulates its proteasomal degradation. To gain further insight into the function of CHIP we isolated CHIP-containing protein complexes from human HeLa cells and analyzed their composition by peptide mass fingerprinting. We identified the Hsc70 cochaperone BAG-2 as a main component of CHIP complexes. BAG-2 inhibits the ubiquitin ligase activity of CHIP by abrogating the CHIP/E2 cooperation and stimulates the chaperone-assisted maturation of CFTR. The activity of BAG-2 resembles that of the previously characterized Hsc70 cochaperone and CHIP inhibitor HspBP1. The presented data therefore establish multiple mechanisms to control the destructive activity of the CHIP ubiquitin ligase in human cells.
Figures




References
-
- Alberti, S., Demand, J., Esser, C., Emmerich, N., Schild, H., and Höhfeld, J. (2002). Ubiquitylation of BAG-1 suggests a novel regulatory mechanism during the sorting of chaperone substrates to the proteasome. J. Biol. Chem. 277, 45920-45927. - PubMed
-
- Ballinger, C. A., Connell, P., Wu, Y., Hu, Z., Thompson, L. J., Yin, L.Y., and Patterson, C. (1999). Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol. Cell. Biol. 19, 4535-4545. - PMC - PubMed
-
- Cardozo, C. P., Michaud, C., Ost, M. C., Fliss, A. E., Yang, E., Patterson, C., Hall, S. J., and Caplan, A. J. (2003). C-terminal Hsp-interacting protein slows androgen receptor synthesis and reduces its rate of degradation. Arch. Biochem. Biophys. 410, 134-140. - PubMed
-
- Connell, P., Ballinger, C. A., Jiang, J., Wu, Y., Thompson, L. J., Höhfeld, J., and Patterson, C. (2001). The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat. Cell Biol. 3, 93-96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

