Beta-amyloid immunotherapy prevents synaptic degeneration in a mouse model of Alzheimer's disease
- PMID: 16207868
- PMCID: PMC6725749
- DOI: 10.1523/JNEUROSCI.1697-05.2005
Beta-amyloid immunotherapy prevents synaptic degeneration in a mouse model of Alzheimer's disease
Abstract
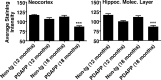
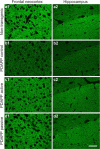
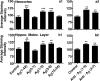
Alzheimer's disease neuropathology is characterized by key features that include the deposition of the amyloid beta peptide (Abeta) into plaques, the formation of neurofibrillary tangles, and the loss of neurons and synapses in specific brain regions. The loss of synapses, and particularly the associated presynaptic vesicle protein synaptophysin in the hippocampus and association cortices, has been widely reported to be one of the most robust correlates of Alzheimer's disease-associated cognitive decline. The beta-amyloid hypothesis supports the idea that Abeta is the cause of these pathologies. However, the hypothesis is still controversial, in part because the direct role of Abeta in synaptic degeneration awaits confirmation. In this study, we show that Abeta reduction by active or passive Abeta immunization protects against the progressive loss of synaptophysin in the hippocampal molecular layer and frontal neocortex of a transgenic mouse model of Alzheimer's disease. These results, substantiated by quantitative electron microscopic analysis of synaptic densities, strongly support a direct causative role of Abeta in the synaptic degeneration seen in Alzheimer's disease and strengthen the potential of Abeta immunotherapy as a treatment approach for this disease.
Figures



References
-
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, et al. (2000) Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 6: 916-919. - PubMed
-
- Bard F, Barbour R, Cannon C, Carretto R, Fox M, Games D, Guido T, Hoenow K, Hu K, Johnson-Wood K, Khan K, Kholodenko D, Lee C, Lee M, Motter R, Nguyen M, Reed A, Schenk D, Tang P, Vasquez N, et al. (2003) Epitope and isotype specificities of antibodies to beta-amyloid peptide for protection against Alzheimer's disease-like neuropathology. Proc Natl Acad Sci USA 100: 2023-2028. - PMC - PubMed
-
- Bussiere T, Bard F, Barbour R, Grajeda H, Guido T, Khan K, Schenk D, Games D, Seubert P, Buttini M (2004) Morphological characterization of thioflavin-S-positive amlyoid plaques in transgenic Alzheimer mice and effect of passive Abeta immunotherapy on their clearance. Am J Pathol 165: 987-995. - PMC - PubMed
-
- Butterfield DA, Bush AI (2004) Alzheimer's amyloid beta-peptide (1-42): involvement of methionine residue 35 in the oxidative stress and neurotoxicity properties of this peptide. Neurobiol Aging 25: 563-568. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
