Early experience modifies the postnatal assembly of autonomic emotional motor circuits in rats
- PMID: 16207869
- PMCID: PMC6725770
- DOI: 10.1523/JNEUROSCI.2345-05.2005
Early experience modifies the postnatal assembly of autonomic emotional motor circuits in rats
Abstract
Rat pups that are repeatedly handled and separated from their dam exhibit altered adult behavioral, endocrine, and autonomic responses to stress, but the extent to which early handling and/or maternal separation (H/S) alters the development of circuits that underlie these responses is unknown. The present study tested the hypothesis that early H/S alters the postnatal assembly of synapses within preautonomic emotional motor circuits. Circuit development was traced by synapse-dependent retrograde transneuronal transport of pseudorabies virus (PRV) from the stomach wall. Control and H/S rats were analyzed between postnatal day 6 (P6) and P10, a period of rapid synaptic assembly among preautonomic circuit components. Pups in H/S groups were removed from their dam daily for either 15 min or 3 h beginning on P1, and were injected with virus on P8 and perfused on P10. Quantitative analyses of primary and transsynaptic PRV immunolabeling confirmed an age-dependent assembly of hypothalamic, limbic, and cortical inputs to autonomic nuclei. Circuit assembly was significantly altered in H/S pups, in which fewer neurons in the central amygdala, the bed nucleus of the stria terminalis, and visceral cortices were infected compared with age-matched controls. In contrast, H/S did not alter the assembly of paraventricular hypothalamic inputs to gastric autonomic neurons. H/S-related reductions in limbic and cortical transneuronal infection were similar in pups exposed daily to 15 min or 3 h maternal separation. These findings support the view that environmental events during early postnatal life can influence the formation of neural circuits that provide limbic and cortical control over autonomic emotional motor output.
Figures


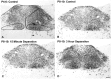

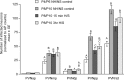



Comment in
-
Early social experience and brain development.J Neurosci. 2006 Feb 15;26(7):1889-90. doi: 10.1523/JNEUROSCI.5133-05.2006. J Neurosci. 2006. PMID: 16481418 Free PMC article. No abstract available.
Similar articles
-
Progressive postnatal assembly of limbic-autonomic circuits revealed by central transneuronal transport of pseudorabies virus.J Neurosci. 2000 Apr 1;20(7):2731-41. doi: 10.1523/JNEUROSCI.20-07-02731.2000. J Neurosci. 2000. PMID: 10729354 Free PMC article.
-
Repeated brief postnatal maternal separation enhances hypothalamic gastric autonomic circuits in juvenile rats.Neuroscience. 2010 Jan 13;165(1):265-77. doi: 10.1016/j.neuroscience.2009.09.081. Epub 2009 Oct 2. Neuroscience. 2010. PMID: 19800939 Free PMC article.
-
Retrograde transynaptic pseudorabies virus infection of central autonomic circuits in neonatal rats.Brain Res Dev Brain Res. 1999 May 14;114(2):207-16. doi: 10.1016/s0165-3806(99)00039-5. Brain Res Dev Brain Res. 1999. PMID: 10320760
-
Use of pseudorabies virus to delineate multisynaptic circuits in brain: opportunities and limitations.J Neurosci Methods. 2000 Nov 15;103(1):51-61. doi: 10.1016/s0165-0270(00)00295-8. J Neurosci Methods. 2000. PMID: 11074095 Review.
-
Transneuronal circuit analysis with pseudorabies viruses.Curr Protoc Neurosci. 2001 May;Chapter 1:Unit1.5. doi: 10.1002/0471142301.ns0105s09. Curr Protoc Neurosci. 2001. PMID: 18428451 Review.
Cited by
-
Repair of the UL21 locus in pseudorabies virus Bartha enhances the kinetics of retrograde, transneuronal infection in vitro and in vivo.J Virol. 2009 Feb;83(3):1173-83. doi: 10.1128/JVI.02102-08. Epub 2008 Nov 19. J Virol. 2009. PMID: 19019952 Free PMC article.
-
Adult depression-like behavior, amygdala and olfactory cortex functions are restored by odor previously paired with shock during infant's sensitive period attachment learning.Dev Cogn Neurosci. 2011 Jan;1(1):77-87. doi: 10.1016/j.dcn.2010.07.005. Dev Cogn Neurosci. 2011. PMID: 21037982 Free PMC article.
-
Programming of Stress-Sensitive Neurons and Circuits by Early-Life Experiences.Front Behav Neurosci. 2019 Feb 18;13:30. doi: 10.3389/fnbeh.2019.00030. eCollection 2019. Front Behav Neurosci. 2019. PMID: 30833892 Free PMC article.
-
Early life stress elicits visceral hyperalgesia and functional reorganization of pain circuits in adult rats.Neurobiol Stress. 2016 Jun 1;3:8-22. doi: 10.1016/j.ynstr.2015.12.003. Neurobiol Stress. 2016. PMID: 26751119 Free PMC article.
-
Progressions on the Coexistence of Neuronal and Glial Precursor Cells in the Cerebral Ventricular Zone.J Neurosci. 2021 Apr 14;41(15):3301-3306. doi: 10.1523/JNEUROSCI.3190-20.2021. Epub 2021 Feb 17. J Neurosci. 2021. PMID: 33597270 Free PMC article. Review.
References
-
- Aleksandrov VG, Bagaev VA, Nozdrachev AD, Panteleev SS (1996) Identification of gastric related neurones in the rat insular cortex. Neurosci Lett 216: 5-8. - PubMed
-
- Andersen SL (2003) Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev 27: 3-18. - PubMed
-
- Armstrong WE, Warach S, Hatton GI, McNeill TH (1980) Subnuclei in the rat hypothalamic paraventricular nucleus: a cytoarchitectural, horseradish peroxidase and immunocytochemical analysis. Neuroscience 5: 1931-1958. - PubMed
-
- Bartha A (1961) Experimental reduction of virulence of Aujeszky's disease virus. Magy Allotorv Lapja 16: 42-45.
-
- Bence M, Levelt CN (2005) Structural plasticity in the developing visual system. Prog Brain Res 147: 125-139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
