Expression and modulation of an NADPH oxidase in mammalian astrocytes
- PMID: 16207877
- PMCID: PMC6725750
- DOI: 10.1523/JNEUROSCI.1632-05.2005
Expression and modulation of an NADPH oxidase in mammalian astrocytes
Abstract
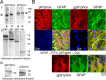
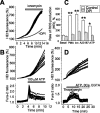
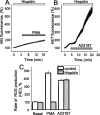
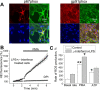
Amyloid beta peptides generate oxidative stress in hippocampal astrocytes through a mechanism sensitive to inhibitors of the NADPH oxidase [diphenylene iodonium (DPI) and apocynin]. Seeking evidence for the expression and function of the enzyme in primary hippocampal astrocytes, we confirmed the expression of the subunits of the phagocyte NADPH oxidase by Western blot analysis and by immunofluorescence and coexpression with the astrocyte-specific marker glial fibrillary acidic protein both in cultures and in vivo. Functional assays using lucigenin luminescence, dihydroethidine, or dicarboxyfluorescein fluorescence to measure the production of reactive oxygen species (ROS) demonstrated DPI and apocynin-sensitive ROS generation in response to the phorbol ester PMA and to raised [Ca2+]c after application of ionomycin or P2u receptor activation. Stimulation by PMA but not Ca2+ was inhibited by the protein kinase C (PKC) inhibitors staurosporine and hispidin. Responses were absent in transgenic mice lacking gp91phox. Expression of gp91phox and p67phox was increased in reactive astrocytes, which showed increased rates of both resting and stimulated ROS generation. NADPH oxidase activity was modulated by intracellular pH, suppressed by intracellular alkalinization, and enhanced by acidification. The protonophore carbonyl cyanide p-trifluoromethoxyphenylhydrazone suppressed basal ROS generation but markedly increased PMA-stimulated ROS generation. This was independent of mitochondrial ROS production, because it was unaffected by mitochondrial depolarization with rotenone and oligomycin. Thus, the NADPH oxidase is expressed in astrocytes and is functional, activated by PKC and intracellular calcium, modulated by pHi, and upregulated by astrocyte activation. The astrocytic NADPH oxidase is likely to play important roles in CNS physiology and pathology.
Figures



References
-
- Abramov AY, Canevari L, Duchen MR (2004b) Calcium signals induced by amyloid β peptide and their consequences in neurons and astrocytes in culture. Biochim Biophys Acta 1742: 81-87. - PubMed
-
- Banfi B, Molnar G, Maturana A, Steger K, Hegedus B, Demaurex N, Krause KH (2001) A Ca2+-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem 276: 37594-38601. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
