Detection of Aspergillus galactomannan antigenemia to determine biological and clinical implications of beta-lactam treatments
- PMID: 16207986
- PMCID: PMC1248458
- DOI: 10.1128/JCM.43.10.5214-5220.2005
Detection of Aspergillus galactomannan antigenemia to determine biological and clinical implications of beta-lactam treatments
Abstract
Detection of Aspergillus galactomannan (GM) in serum with the Platelia Aspergillus enzyme immunoassay (EIA) is useful for diagnosing invasive aspergillosis. From May 2003 to November 2004, 65 patients who did not develop aspergillosis had at least two positive sera while receiving a beta-lactam treatment (GM index [GMI], >or=0.5). Of the 69 treatment episodes scored, 41 consisted of a beta-lactam other than piperacillin-tazobactam (n=29), namely, amoxicillin-clavulanate (n=25), amoxicillin (n=10), ampicillin (n=3), or phenoxymethylpenicillin (n=2). In all cases, antigenemia became negative 24 h to 120 h upon stopping the antibiotic. Monitoring of 35 patients, including 26 with hematological malignancies, revealed three antigenemia kinetic patterns: each was observed with any drug regimen and consisted of a persistent GMI of >2.0 (65.7%), >0.5, and <or=1.5 (25.7%) or a variable GMI (14.3%) from the onset of antibiotic therapy. All available drug batches given to 26 patients cross-reacted with the EIA. Galactomannan titration in batches failed to predict the GM titers in the five patients studied at cumulative doses of ampicillin or amoxicillin-clavulanate, regardless of the time lapse between serum sampling and infusion period. Our results show that beta-lactams other than piperacillin-tazobactam may lead to false presumption of aspergillosis. The resulting kinetic patterns of GM antigenemia are variable, and sampling serum prior to the next beta-lactam dose may not decrease GMI below the threshold. Consequently, testing of suspected antibiotic batches remains the only indicator of possible false EIA positivity.
Figures

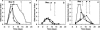
References
-
- Adam, O., A. Auperin, F. Wilquin, J.-H. Bourhis, B. Gachot, and E. Chachaty. 2004. Treatment with piperacillin-tazobactam and false-positive Aspergillus galactomannan antigen test results for patients with hematological malignancies. Clin. Infect. Dis. 38:917-920. - PubMed
-
- Ansorg, R., R. van den Boom, and P. M. Rath. 1997. Detection of Aspergillus galactomannan antigen in foods and antibiotics. Mycoses 40:353-357. - PubMed
-
- Ascioglu, S., J. H. Rex, J. E. Bennett, J. Billie, F. Croksert, D. W. Denning, J. P. Donnelly, J. E. Edwards, Z. Erjavec, D. Fiere, O. Lortholary, J. Maertens, J. F. Meis, T. F. Patterson, J. Ritter, D. Selleslag, P. M. Shah, D. A. Stevens, and J. Walsh. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7-14. - PubMed
-
- Bennett, J. E., M. M. Friedman, and B. Dupont. 1987. Receptor-mediated clearance of Aspergillus galactomannan. J. Infect. Dis. 155:1005-1010. - PubMed
-
- Boutboul, F., C. Albert, T. Leblan, A. Sulahia, E. Gluckman, F. Derouin, and P. Ribaud. 2002. Invasive aspergillosis in allogeneic stem cell transplant recipients: increasing antigenemia is associated with progressive disease. Clin. Infect. Dis. 34:939-943. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

