Exercise rapidly increases eukaryotic elongation factor 2 phosphorylation in skeletal muscle of men
- PMID: 16210351
- PMCID: PMC1464212
- DOI: 10.1113/jphysiol.2005.097154
Exercise rapidly increases eukaryotic elongation factor 2 phosphorylation in skeletal muscle of men
Abstract
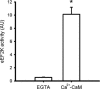
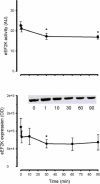
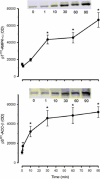
Protein synthesis in skeletal muscle is known to decrease during contractions but the underlying regulatory mechanisms are unknown. Here, the effect of exercise on skeletal muscle eukaryotic elongation factor 2 (eEF2) phosphorylation, a key component in protein translation machinery, was examined. Eight healthy men exercised on a cycle ergometer at a workload eliciting approximately 67% peak pulmonary oxygen consumption (VO2 peak) with skeletal muscle biopsies taken from the vastus lateralis muscle at rest as well as after 1, 10, 30, 60 and 90 min of exercise. In response to exercise, there was a rapid (i.e. < 1 min) 5- to 7-fold increase in eEF2 phosphorylation at Thr56 that was sustained for 90 min of continuous exercise. The in vitro activity of skeletal muscle eEF2 kinase was not altered by exercise indicating that the increased activity of eEF2 kinase to eEF2 is not mediated by covalent mechanisms. In support of this, the increase in AMPK activity was temporally unrelated to eEF2 phosphorylation. However, skeletal muscle eEF2 kinase was potently activated by Ca(2)(+)-calmodulin in vitro, suggesting that the higher eEF2 phosphorylation in working skeletal muscle is mediated by allosteric activation of eEF2 kinase by Ca(2)(+) signalling via calmodulin. Given that eEF2 phosphorylation inhibits eEF2 activity and mRNA translation, these findings suggest that the inhibition of protein synthesis in contracting skeletal muscle is due to the Ca(2)(+)-induced stimulation of eEF2 kinase.
Figures

Comment in
-
Why muscle stops building when it's working.J Physiol. 2005 Nov 15;569(Pt 1):3. doi: 10.1113/jphysiol.2005.099424. Epub 2005 Oct 6. J Physiol. 2005. PMID: 16210346 Free PMC article. No abstract available.
References
-
- Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H. Selective activation of AMPK-PGC-1α or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J. 2005;19:786–788. - PubMed
-
- Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. - PubMed
-
- Bolster DR, Jefferson LS, Kimball SR. Regulation of protein synthesis associated with skeletal muscle hypertrophy by insulin-, amino acid- and exercise-induced signalling. Proc Nutr Soc. 2004;63:351–356. - PubMed
-
- Browne GJ, Finn SG, Proud CG. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J Biol Chem. 2004;279:12220–12231. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

