Hyperhomocystinemia impairs endothelial function and eNOS activity via PKC activation
- PMID: 16210565
- PMCID: PMC4400833
- DOI: 10.1161/01.ATV.0000189559.87328.e4
Hyperhomocystinemia impairs endothelial function and eNOS activity via PKC activation
Abstract
Objective: A risk factor for cardiovascular disease, hyperhomocystinemia (HHcy), is associated with endothelial dysfunction. In this study, we examined the mechanistic role of HHcy in endothelial dysfunction.
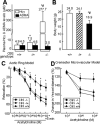
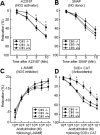
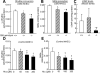
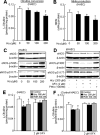
Methods and results: Through the use of 2 functional models, aortic rings and intravital video microscopy of the cremaster, we found that arterial relaxation in response to the endothelium-dependent vessel relaxant, acetylcholine or the nitric oxide synthase (NOS) activator (A23187), was significantly impaired in cystathionine beta-synthase null (CBS(-/-)) mice. However, the vascular smooth muscle cell (VSMC) response to the nitric oxide (NO) donor (SNAP) was preserved in CBS(-/-) mice. In addition, superoxide dismutase and catalase failed to restore endothelium-dependent vasodilatation. Endothelial nitric oxide synthase (eNOS) activity was significantly reduced in mouse aortic endothelial cells (MAECs) of CBS(-/-) mice, as well as in Hcy-treated mouse and human aortic endothelial cells (HAECs). Hcy-mediated eNOS inhibition--which was not rescued by adenoviral transduction of superoxide dismutase and glutathione peroxidase, or by tetrahydrobiopterin, sepiapterin, and arginine supplementations in MAEC--was associated with decreased protein expression and increased threonine 495 phosphorylation of eNOS in HAECs. Ultimately, a protein kinase C (PKC) inhibitor, GF109203X (GFX), reversed Hcy-mediated eNOS inactivation and threonine 495 phosphorylation in HAECs.
Conclusions: These data suggest that HHcy impairs endothelial function and eNOS activity, primarily through PKC activation.
Figures


References
-
- Wang H, Tan H, Yang F. Mechanisms in homocysteine-induced vascular disease. Drug Discovery Today (Disease mechanisms) 2005;2:25–31.
-
- Yang F, Tan HM, Wang H. Hyperhomocysteinemia and atherosclerosis. Acta Physiologica Sinica. 2005;57:103–114. - PubMed
-
- Schulz E, Anter E, Keaney JF., Jr Oxidative stress, antioxidants, and endothelial function. Curr Med Chem. 2004;11:1093–1104. - PubMed
-
- Tawakol A, Omland T, Gerhard M, Wu JT, Creager MA. Hyperhomocyst(e)inemia is associated with impaired endothelium-dependent vasodilation in humans. Circulation. 1997;95:1119–1121. - PubMed
-
- Bennett-Richards K, Kattenhorn M, Donald A, Oakley G, Varghese Z, Rees L, Deanfield JE. Does oral folic acid lower total homocysteine levels and improve endothelial function in children with chronic renal failure? Circulation. 2002;105:1810–1815. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

