Propensity of adult lymphoid progenitors to progress to DN2/3 stage thymocytes with Notch receptor ligation
- PMID: 16210587
- PMCID: PMC1850239
- DOI: 10.4049/jimmunol.175.8.4858
Propensity of adult lymphoid progenitors to progress to DN2/3 stage thymocytes with Notch receptor ligation
Abstract
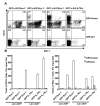
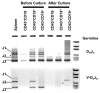
Notch family receptors control critical events in the production and replenishment of specialized cells in the immune system. However, it is unclear whether Notch signaling regulates abrupt binary lineage choices in homogeneous progenitors or has more gradual influence over multiple aspects of the process. A recently developed coculture system with Delta 1-transduced stromal cells is being extensively used to address such fundamental questions. Different from fetal progenitors, multiple types of adult marrow cells expanded indefinitely in murine Delta-like 1-transduced OP9 cell cocultures, progressed to a DN2/DN3 thymocyte stage, and slowly produced TCR(+) and NK cells. Long-term cultured cells of this kind retained some potential for T lymphopoiesis in vivo. Adult marrow progressed through double-positive and single-positive stages only when IL-7 concentrations were low and passages were infrequent. Lin(-)c-Kit(low)GFP(+)IL-7Ralpha(+/-) prolymphocytes were the most efficient of adult bone marrow cells in short-term cultures, but the assay does not necessarily reflect cells normally responsible for replenishing the adult thymus. Although marrow-derived progenitors with Ig D(H)-J(H) rearrangements acquired T lineage characteristics in this model, that was not the case for more B committed cells with V(H)-D(H)J(H) rearrangement products.
Figures





Similar articles
-
Fc gamma RII/III and CD2 expression mark distinct subpopulations of immature CD4-CD8- murine thymocytes: in vivo developmental kinetics and T cell receptor beta chain rearrangement status.J Exp Med. 1993 Apr 1;177(4):1079-92. doi: 10.1084/jem.177.4.1079. J Exp Med. 1993. PMID: 8096236 Free PMC article.
-
Analysis of Notch1 function by in vitro T cell differentiation of Pax5 mutant lymphoid progenitors.J Immunol. 2004 Sep 15;173(6):3935-44. doi: 10.4049/jimmunol.173.6.3935. J Immunol. 2004. PMID: 15356142
-
Cellular and molecular requirements for the selection of in vitro-generated CD8 T cells reveal a role for Notch.J Immunol. 2013 Aug 15;191(4):1704-15. doi: 10.4049/jimmunol.1300417. Epub 2013 Jul 12. J Immunol. 2013. PMID: 23851691 Free PMC article.
-
Fidelity and infidelity in commitment to B-lymphocyte lineage development.Immunol Rev. 2000 Jun;175:104-11. Immunol Rev. 2000. PMID: 10933595 Review.
-
T-sing progenitors to commit.Trends Immunol. 2006 Mar;27(3):125-31. doi: 10.1016/j.it.2006.01.006. Epub 2006 Feb 10. Trends Immunol. 2006. PMID: 16473042 Review.
Cited by
-
Competition and collaboration: GATA-3, PU.1, and Notch signaling in early T-cell fate determination.Semin Immunol. 2008 Aug;20(4):236-46. doi: 10.1016/j.smim.2008.07.006. Epub 2008 Sep 3. Semin Immunol. 2008. PMID: 18768329 Free PMC article. Review.
-
Cell cycle quiescence of early lymphoid progenitors in adult bone marrow.Stem Cells. 2006 Dec;24(12):2703-13. doi: 10.1634/stemcells.2006-0217. Epub 2006 Aug 24. Stem Cells. 2006. PMID: 16931772 Free PMC article.
-
Functional diversity of stem and progenitor cells with B-lymphopoietic potential.Immunol Rev. 2010 Sep;237(1):10-21. doi: 10.1111/j.1600-065X.2010.00933.x. Immunol Rev. 2010. PMID: 20727026 Free PMC article. Review.
-
Vitamin C promotes maturation of T-cells.Antioxid Redox Signal. 2013 Dec 10;19(17):2054-67. doi: 10.1089/ars.2012.4988. Epub 2013 Feb 5. Antioxid Redox Signal. 2013. PMID: 23249337 Free PMC article.
-
Primitive lymphoid progenitors in bone marrow with T lineage reconstituting potential.J Immunol. 2006 Sep 1;177(5):2880-7. doi: 10.4049/jimmunol.177.5.2880. J Immunol. 2006. PMID: 16920923 Free PMC article.
References
-
- Maillard I, Adler SH, Pear WS. Notch and the immune system. Immunity. 2003;19:781–791. - PubMed
-
- Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, Aguet M. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. - PubMed
-
- Pui JC, Allman D, Xu L, DeRocco S, Karnell FG, Bakkour S, Lee JY, Kadesch T, Hardy RR, Aster JC, Pear WS. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11:299–308. - PubMed
-
- Schmitt TM, Zúñiga-Pflücker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG034876/AG/NIA NIH HHS/United States
- P20 RR15577/RR/NCRR NIH HHS/United States
- AI20069/AI/NIAID NIH HHS/United States
- R21 AI053739/AI/NIAID NIH HHS/United States
- P20 RR015577/RR/NCRR NIH HHS/United States
- R01 AI058162/AI/NIAID NIH HHS/United States
- AI53739/AI/NIAID NIH HHS/United States
- R56 AI033940/AI/NIAID NIH HHS/United States
- R01 AI033940/AI/NIAID NIH HHS/United States
- R29 AI033940/AI/NIAID NIH HHS/United States
- AI58162/AI/NIAID NIH HHS/United States
- AI33940/AI/NIAID NIH HHS/United States
- R21 AI033940/AI/NIAID NIH HHS/United States
- R01 AI020069/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

