Growth of pulmonary microvasculature in ventilated preterm infants
- PMID: 16210670
- PMCID: PMC2662989
- DOI: 10.1164/rccm.200506-927OC
Growth of pulmonary microvasculature in ventilated preterm infants
Abstract
Rationale: Density-based morphometric studies have demonstrated decreased capillary density in infants with bronchopulmonary dysplasia (BPD) and in BPD-like animal models, leading to the prevailing view that microvascular development is disrupted in BPD.
Objective: To perform a comprehensive analysis of the early and late effects of ventilation on pulmonary microvascular growth in preterm infants.
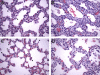
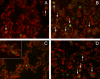
Methods: Postmortem lung samples were collected from ventilated preterm infants who died between 23 and 29 wk ("short-term ventilated") or between 36 and 39 wk ("long-term ventilated") corrected postmenstrual age. Results were compared with age-matched infants or stillborn infants ("early" and "late" control subjects). Microvascular growth was studied by anti-platelet endothelial cell adhesion molecule (PECAM)-1 immunohistochemistry, quantitative stereology, analysis of endothelial cell proliferation, and Western blot analysis of pulmonary PECAM-1 protein levels.
Measurements: Measurements were made of capillary density, volume of air-exchanging parenchyma, volume of microvascular endothelial cells, Ki67 labeling index of endothelial cells, and PECAM-1/actin protein levels.
Main results: Lungs of long-term ventilated infants showed a significant (more than twofold) increase in volume of air-exchanging parenchyma and a 60% increase in total pulmonary microvascular endothelial volume compared with late control subjects, associated with 60% higher pulmonary PECAM-1 protein levels. The marked expansion of the pulmonary microvasculature in ventilated lungs was, at least partly, attributable to brisk endothelial cell proliferation. The microvasculature of ventilated lungs appeared immature, retaining a saccular architectural pattern.
Conclusions: The pulmonary microvasculature of ventilated preterm infants displayed marked angiogenesis, nearly proportionate to the growth of the air-exchanging lung parenchyma. These results challenge the paradigm of microvascular growth arrest as a major pathogenic factor in BPD.
Figures




References
-
- Jobe AH, Ikegami M. Mechanisms initiating lung injury in the preterm. Early Hum Dev 1998;53:81–94. - PubMed
-
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–1729. - PubMed
-
- Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, Verter J, Temprosa M, Wright LL, Ehrenkranz RA, et al.; NICHD Neonatal Research Network. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1995 through December 1996. Pediatrics 2001;107:E1. - PubMed
-
- Bonikos DS, Bensch KG, Northway WH Jr, Edwards DK. Bronchopulmonary dysplasia: the pulmonary pathologic sequel of necrotizing bronchiolitis and pulmonary fibrosis. Hum Pathol 1976;7:643–666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

