Pemphigus foliaceus IgG causes dissociation of desmoglein 1-containing junctions without blocking desmoglein 1 transinteraction
- PMID: 16211092
- PMCID: PMC1242188
- DOI: 10.1172/JCI23475
Pemphigus foliaceus IgG causes dissociation of desmoglein 1-containing junctions without blocking desmoglein 1 transinteraction
Abstract
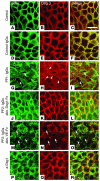
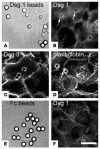
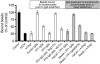
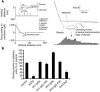
Autoantibodies against the epidermal desmosomal cadherins desmoglein 1 (Dsg1) and Dsg3 have been shown to cause severe to lethal skin blistering clinically defined as pemphigus foliaceus (PF) and pemphigus vulgaris (PV). It is unknown whether antibody-induced dissociation of keratinocytes is caused by direct inhibition of Dsg1 transinteraction or by secondary cellular responses. Here we show in an in vitro system that IgGs purified from PF patient sera caused cellular dissociation of cultured human keratinocytes as well as significant release of Dsg1-coated microbeads attached to Dsg-containing sites on the keratinocyte cellular surface. However, cell dissociation and bead release induced by PF-IgGs was not caused by direct steric hindrance of Dsg1 transinteraction, as demonstrated by single molecule atomic force measurements and by laser trapping of surface-bound Dsg1-coated microbeads. Rather, our experiments strongly indicate that PF-IgG-mediated dissociation events must involve autoantibody-triggered cellular signaling pathways, resulting in destabilization of Dsg1-based adhesive sites and desmosomes.
Figures



Republished in
-
Wolfgang Bargmann-Preis 2006. Pemphigus foliaceus igG causes dissociation of desmoglein 1-containing junctions without blocking desmoglein 1 transinteraction.Ann Anat. 2006 Nov;188(6):501-2. doi: 10.1016/j.aanat.2006.05.012. Ann Anat. 2006. PMID: 17140142 No abstract available.
References
-
- Amagai M, Hashimoto T, Green KJ, Shimizu N, Nishikawa T. Antigen-specific immunoadsorption of pathogenic autoantibodies in pemphigus foliaceus. J. Invest. Dermatol. 1995;104:895–901. - PubMed
-
- Nollet F, Kools P, van Roy F. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J. Mol. Biol. 2000;299:551–572. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

