Modulation of atherosclerosis in mice by Toll-like receptor 2
- PMID: 16211093
- PMCID: PMC1242192
- DOI: 10.1172/JCI25482
Modulation of atherosclerosis in mice by Toll-like receptor 2
Abstract
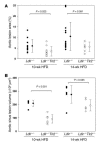
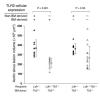
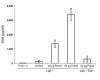
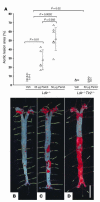
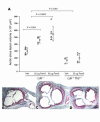
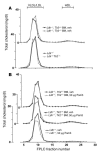
Epidemiologic evidence has established a relationship between microbial infection and atherosclerosis. Mammalian TLRs provide clues on the mechanism of this inflammatory cascade. TLR2 has a large ligand repertoire that includes bacterial-derived exogenous and possibly host-derived endogenous ligands. In atherosclerosis-susceptible low-density lipoprotein receptor-deficient (Ldlr-/-) mice, complete deficiency of TLR2 led to a reduction in atherosclerosis. However, with BM transplantation, loss of TLR2 expression from BM-derived cells had no effect on disease progression. This suggested that an unknown endogenous TLR2 agonist influenced lesion progression by activating TLR2 in cells that were not of BM cell origin. Moreover, with intraperitoneal administration of a synthetic TLR2/TLR1 agonist, Pam3CSK4, disease burden was dramatically increased in Ldlr-/- mice. A complete deficiency of TLR2 in Ldlr-/- mice, as well as a deficiency of TLR2 only in BM-derived cells in Ldlr-/- mice, led to striking protection against Pam3CSK4-mediated atherosclerosis, suggesting a role for BM-derived cell expression of TLR2 in transducing the effects of an exogenous TLR2 agonist. These studies support the concept that chronic or recurrent microbial infections may contribute to atherosclerotic disease. Additionally, these data suggest the presence of host-derived endogenous TLR2 agonists.
Figures

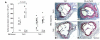
References
-
- Murray CJ, et al. Effectiveness and costs of interventions to lower systolic blood pressure and cholesterol: a global and regional analysis on reduction of cardiovascular-disease risk. Lancet. 2003;361:717–725. - PubMed
-
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. - PubMed
-
- Kol A, Libby P. The mechanisms by which infectious agents may contribute to atherosclerosis and its clinical manifestations. Trends Cardiovasc. Med. 1998;8:191–199. - PubMed
-
- Mendall MA, et al. Chlamydia pneumoniae: risk factors for seropositivity and association with coronary heart disease. J. Infect. 1995;30:121–128. - PubMed
-
- Kalayoglu MV, Libby P, Byrne GI. Chlamydia pneumoniae as an emerging risk factor in cardiovascular disease. JAMA. 2002;288:2724–2731. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

