Identification and analysis of multivalent proteolytically resistant peptides from gluten: implications for celiac sprue
- PMID: 16212427
- PMCID: PMC1343496
- DOI: 10.1021/pr050173t
Identification and analysis of multivalent proteolytically resistant peptides from gluten: implications for celiac sprue
Abstract
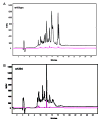
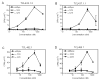
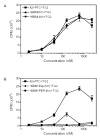
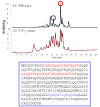
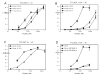
Dietary gluten proteins from wheat, rye, and barley are the primary triggers for the immuno-pathogenesis of Celiac Sprue, a widespread immune disease of the small intestine. Recent molecular and structural analyses of representative gluten proteins, most notably alpha- and gamma-gliadin proteins from wheat, have improved our understanding of these pathogenic mechanisms. In particular, based on the properties of a 33-mer peptide, generated from alpha-gliadin under physiological conditions, a link between digestive resistance and inflammatory character of gluten has been proposed. Here, we report three lines of investigation in support of this hypothesis. First, biochemical and immunological analysis of deletion mutants of alpha-2 gliadin confirmed that the DQ2 restricted T cell response to the alpha-2 gliadin are directed toward the epitopes clustered within the 33-mer. Second, proteolytic analysis of a representative gamma-gliadin led to the identification of another multivalent 26-mer peptide that was also resistant to further gastric, pancreatic and intestinal brush border degradation, and was a good substrate of human transglutaminase 2 (TG2). Analogous to the 33-mer, the synthetic 26-mer peptide displayed markedly enhanced T cell antigenicity compared to monovalent control peptides. Finally, in silico analysis of the gluten proteome led to the identification of at least 60 putative peptides that share the common characteristics of the 33-mer and the 26-mer peptides. Together, these results highlight the pivotal role of physiologically generated, proteolytically stable, TG2-reactive, multivalent peptides in the immune response to dietary gluten in Celiac Sprue patients. Prolyl endopeptidase treatment was shown to abolish the antigenicity of both the 33-mer and the 26-mer peptides, and was also predicted to have comparable effects on other proline-rich putatively immunotoxic peptides identified from other polypeptides within the gluten proteome.
Figures

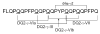
References
-
- Schuppan D. Current concepts of celiac disease pathogenesis. Gastroenterology. 2000;119(1):234–42. - PubMed
-
- Sollid LM. Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol. 2002;2(9):647–55. - PubMed
-
- Sollid LM. Molecular basis of celiac disease. Annu Rev Immunol. 2000;18:53–81. - PubMed
-
- Maki M, Collin P. Coeliac disease. Lancet. 1997;349(9067):1755–9. - PubMed
-
- Corrao G, Corazza GR, Bagnardi V, Brusco G, Ciacci C, Cottone M, Sategna Guidetti C, Usai P, Cesari P, Pelli MA, Loperfido S, Volta U, Calabro A, Certo M. Mortality in patients with coeliac disease and their relatives: a cohort study. Lancet. 2001;358(9279):356–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

