Asthma and COPD in cystic fibrosis intron-8 5T carriers. A population-based study
- PMID: 16212675
- PMCID: PMC1277850
- DOI: 10.1186/1465-9921-6-113
Asthma and COPD in cystic fibrosis intron-8 5T carriers. A population-based study
Abstract
Background: Carriers of cystic fibrosis intron-8 5T alleles with high exon-9 skipping could have increased annual lung function decline and increased risk for asthma or chronic obstructive pulmonary disease (COPD).
Methods: We genotyped 9131 individuals from the adult Danish population for cystic fibrosis 5T, 7T, 9T, and F508del alleles, and examined associations between 11 different genotype combinations, and annual FEV1 decline and risk of asthma or COPD.
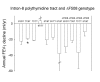
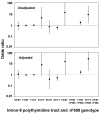
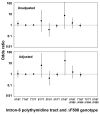
Results: 5T heterozygotes vs. 7T homozygous controls had no increase in annual FEV1 decline, self-reported asthma, spirometry-defined COPD, or incidence of hospitalization from asthma or COPD. In 5T/7T heterozygotes vs. 7T homozygous controls we had 90% power to detect an increase in FEV1 decline of 8 ml, an odds ratio for self-reported asthma and spirometry-defined COPD of 1.9 and 1.7, and a hazard ratio for asthma and COPD hospitalization of 1.8 and 1.6, respectively. Both 5T homozygotes identified in the study showed evidence of asthma, while none of four 5T/F508del compound heterozygotes had severe pulmonary disease. 7T/9T individuals had annual decline in FEV1 of 19 ml compared with 21 ml in 7T homozygous controls (t-test: P = 0.03). 6.7% of 7T homozygotes without an F508del allele in the cystic fibrosis transmembrane conductance regulator gene reported asthma vs. 11% of 7T/9T individuals with an F508del allele (chi2: P = 0.01) and 40% of 7T homozygotes with an F508del allele (P = 0.04). 7T homozygotes with vs. without an F508del allele also had higher incidence of asthma hospitalization (log-rank: P = 0.003); unadjusted and adjusted equivalent hazard ratios for asthma hospitalization were 11 (95%CI: 1.5-78) and 6.3 (0.84-47) in 7T homozygotes with vs. without an F508del allele.
Conclusion: Polythymidine 5T heterozygosity is not associated with pulmonary dysfunction or disease in the adult Caucasian population. Furthermore, our results support that F508del heterozygosity is associated with increased asthma risk independently of the 5T allele.
Figures
Similar articles
-
Genetic and biochemical markers of obstructive lung disease in the general population.Clin Respir J. 2009 Apr;3(2):121-2. doi: 10.1111/j.1752-699X.2008.00110.x. Clin Respir J. 2009. PMID: 20298391
-
Fifteen-year follow-up of pulmonary function in individuals heterozygous for the cystic fibrosis phenylalanine-508 deletion.J Allergy Clin Immunol. 2001 May;107(5):818-23. doi: 10.1067/mai.2001.114117. J Allergy Clin Immunol. 2001. PMID: 11344348
-
β2-adrenergic receptor polymorphisms, asthma and COPD: two large population-based studies.Eur Respir J. 2012 Mar;39(3):558-66. doi: 10.1183/09031936.00023511. Epub 2011 Nov 10. Eur Respir J. 2012. PMID: 22075484
-
The protease inhibitor PI*S allele and COPD: a meta-analysis.Eur Respir J. 2005 Jul;26(1):67-76. doi: 10.1183/09031936.05.00135704. Eur Respir J. 2005. PMID: 15994391 Review.
-
Asthma and COPD in alpha-1 antitrypsin deficiency. Evidence for the Dutch hypothesis.COPD. 2010 Oct;7(5):366-74. doi: 10.3109/15412555.2010.510159. COPD. 2010. PMID: 20854052 Review.
Cited by
-
Relationships among CFTR expression, HCO3- secretion, and host defense may inform gene- and cell-based cystic fibrosis therapies.Proc Natl Acad Sci U S A. 2016 May 10;113(19):5382-7. doi: 10.1073/pnas.1604905113. Epub 2016 Apr 25. Proc Natl Acad Sci U S A. 2016. PMID: 27114540 Free PMC article.
-
An in silico framework for integrating epidemiologic and genetic evidence with health care applications: ventilation-related pneumothorax as a case illustration.J Am Med Inform Assoc. 2016 Jul;23(4):711-20. doi: 10.1093/jamia/ocw031. Epub 2016 Apr 23. J Am Med Inform Assoc. 2016. PMID: 27107435 Free PMC article.
-
Critical modifier role of membrane-cystic fibrosis transmembrane conductance regulator-dependent ceramide signaling in lung injury and emphysema.J Immunol. 2011 Jan 1;186(1):602-13. doi: 10.4049/jimmunol.1002850. Epub 2010 Dec 6. J Immunol. 2011. PMID: 21135173 Free PMC article.
-
Markers of early disease and prognosis in COPD.Int J Chron Obstruct Pulmon Dis. 2009;4:157-67. doi: 10.2147/copd.s3106. Epub 2009 Apr 15. Int J Chron Obstruct Pulmon Dis. 2009. PMID: 19436688 Free PMC article. Review.
-
The role of CFTR mutations in asthma.Can Respir J. 2012 Jan-Feb;19(1):44-5. doi: 10.1155/2012/267251. Can Respir J. 2012. PMID: 22332134 Free PMC article. No abstract available.
References
-
- Tzetis M, Efthymiadou A, Strofalis S, Psychou P, Dimakou A, Pouliou E, et al. CFTR gene mutations – including three novel nucleotide substitutions – and haplotype background in patients with asthma, disseminated brochiectasis and chronic obstructive pulmonary disease. Hum Genet. 2001;108:216–221. doi: 10.1007/s004390100467. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

