Successful production of pseudotyped rAAV vectors using a modified baculovirus expression system
- PMID: 16213797
- PMCID: PMC1351154
- DOI: 10.1016/j.ymthe.2005.08.018
Successful production of pseudotyped rAAV vectors using a modified baculovirus expression system
Abstract
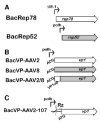
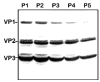
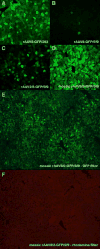
Scalable production of rAAV vectors remains a major obstacle to the clinical application of this prototypical gene therapy vector. A recently developed baculovirus-based production protocol (M. Urabe et al., 2002, Hum. Gene Ther. 13, 1935-1943) found limited applications due to the system's design. Here we report a detailed analysis of the stability of the original baculovirus system components BacRep, BacVP, and transgene cassette-containing BacGFP. All of the baculovirus helpers analyzed were prone to passage-dependent loss-of-function deletions resulting in considerable decreases in rAAV titers. To alleviate the instability and to extend the baculovirus platform to other rAAV serotypes, we have modified both Rep- and Cap-encoding components of the original system. The modifications include a parvoviral phospholipase A2 domain swap allowing production of infectious rAAV8 vectors in vivo. Alternatively, an infectious rAAV8 (or rAAV5) vector incorporating the AAV2 VP1 capsid protein in a mosaic vector particle with AAV8 capsid proteins was produced using a novel baculovirus vector. In this vector, the level of AAV2 VP1 expression is controlled with a "riboswitch," a self-cleaving ribozyme controlled by toyocamycin in the "ON" mode. The redesigned baculovirus system improves our capacity for rAAV manufacturing by making this production platform more applicable to other existing serotypes.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

