Mechanical resistance of proteins explained using simple molecular models
- PMID: 16214858
- PMCID: PMC1367027
- DOI: 10.1529/biophysj.105.071035
Mechanical resistance of proteins explained using simple molecular models
Abstract
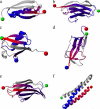
Recent experiments have demonstrated that proteins unfold when two atoms are mechanically pulled apart, and that this process is different to when heated or when a chemical denaturant is added to the solution. Experiments have also shown that the response of proteins to external forces is very diverse, some of them being "hard," and others "soft." Mechanical resistance originates from the presence of barriers on the energy landscape; together, experiment and simulation have demonstrated that unfolding occurs through alternative pathways when different pairs of atoms undergo mechanical extension. Here we use simulation to probe the mechanical resistance of six structurally diverse proteins when pulled in different directions. For this, we use two very different models: a detailed, transferable one, and a coarse-grained, structure-based one. The coarse-grained model gives results that are surprisingly similar to the detailed one and qualitatively agree with experiment; i.e., the mechanical resistance of different proteins or of a single protein pulled in different directions can be predicted by simulation. The results demonstrate the importance of pulling direction relative to the local topology in determining mechanical stability, and rationalize the effect of the location of importation/degradation tags on the rates of mitochondrial import or protein degradation in vivo.
Figures


References
-
- Rief, M., M. Gautel, F. Oesterhelt, J. M. Fernandez, and H. E. Gaub. 1997. Reversible unfolding of individual titin immunoglobulin domains by AFM. Science. 276:1109–1112. - PubMed
-
- Fowler, S., R. B. Best, J. L. Toca-Herrera, T. Rutherford, A. Steward, E. Paci, M. Karplus, and J. Clarke. 2002. Mechanical unfolding of a titin Ig domain: structure of unfolding intermediate revealed by combining AFM, molecular dynamics simulations, NMR and protein engineering. J. Mol. Biol. 322:841–849. - PubMed
-
- Brockwell, D. J., E. Paci, R. C. Zinober, G. S. Beddard, P. D. Olmsted, D. A. Smith, R. N. Perham, and S. E. Radford. 2003. Pulling geometry defines the mechanical resistance of a β-sheet protein. Nat. Struct. Biol. 10:731–737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

