Quantification of calcium entry at the T-tubules and surface membrane in rat ventricular myocytes
- PMID: 16214862
- PMCID: PMC1367035
- DOI: 10.1529/biophysj.105.069013
Quantification of calcium entry at the T-tubules and surface membrane in rat ventricular myocytes
Abstract
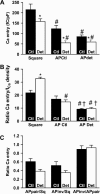
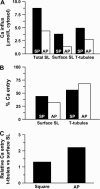
The action potential of cardiac ventricular myocytes is characterized by its long duration, mainly due to Ca flux through L-type Ca channels. Ca entry also serves to trigger the release of Ca from the sarcoplasmic reticulum. The aim of this study was to investigate the role of cell membrane invaginations called transverse (T)-tubules in determining Ca influx and action potential duration in cardiac ventricular myocytes. We used the whole cell patch clamp technique to record electrophysiological activity in intact rat ventricular myocytes (i.e., from the T-tubules and surface sarcolemma) and in detubulated myocytes (i.e., from the surface sarcolemma only). Action potentials were significantly shorter in detubulated cells than in control cells. In contrast, resting membrane potential and action potential amplitude were similar in control and detubulated myocytes. Experiments under voltage clamp using action potential waveforms were used to quantify Ca entry via the Ca current. Ca entry after detubulation was reduced by approximately 60%, a value similar to the decrease in action potential duration. We calculated that Ca influx at the T-tubules is 1.3 times that at the cell surface (4.9 vs. 3.8 micromol/L cytosol, respectively) during a square voltage clamp pulse. In contrast, during a cardiac action potential, Ca entry at the T-tubules is 2.2 times that at the cell surface (3.0 vs. 1.4 micromol/L cytosol, respectively). However, more Ca entry occurs per microm(2) of junctional membrane at the cell surface than in the T-tubules (in nM/microm(2): 1.43 vs. 1.06 during a cardiac action potential). This difference is unlikely to be due to a difference in the number of Ca channels/junction at each site because we estimate that the same number of Ca channels is present at cell surface and T-tubule junctions ( approximately 35). This study provides the first evidence that the T-tubules are a key site for the regulation of action potential duration in ventricular cardiac myocytes. Our data also provide the first direct measurements of T-tubular Ca influx, which are consistent with the idea that cardiac excitation-contraction coupling largely occurs at the T-tubule dyadic clefts.
Figures



References
-
- Wang, S. Q., L. S. Song, E. G. Lakatta, and H. Cheng. 2001. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature. 410:592–596. - PubMed
-
- Wier, W. G., and C. W. Balke. 1999. Ca2+ release mechanisms, Ca2+ sparks, and local control of excitation-contraction coupling in normal heart muscle. Circ. Res. 85:770–776. - PubMed
-
- Cannell, M. B., J. R. Berlin, and W. J. Lederer. 1987. Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science. 238:1419–1423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

