Lipid asymmetry in DLPC/DSPC-supported lipid bilayers: a combined AFM and fluorescence microscopy study
- PMID: 16214871
- PMCID: PMC1367021
- DOI: 10.1529/biophysj.105.067066
Lipid asymmetry in DLPC/DSPC-supported lipid bilayers: a combined AFM and fluorescence microscopy study
Abstract
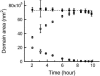
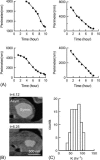
A fundamental attribute of cell membranes is transmembrane asymmetry, specifically the formation of ordered phase domains in one leaflet that are compositionally different from the opposing leaflet of the bilayer. Using model membrane systems, many previous studies have demonstrated the formation of ordered phase domains that display complete transmembrane symmetry; but there have been few reports on the more biologically relevant asymmetric membrane structures. Here we report on a combined atomic force microscopy and fluorescence microscopy study whereby we observe three different states of transmembrane symmetry in phase-separated supported lipid bilayers formed by vesicle fusion. We find that if the leaflets differ in gel-phase area fraction, then the smaller domains in one leaflet are in registry with the larger domains in the other leaflet and the system is dynamic. In a presumed lipid flip-flop process similar to Ostwald ripening, the smaller domains in one leaflet erode away whereas the large domains in the other leaflet grow until complete compositional asymmetry is reached and remains stable. We have quantified this evolution and determined that the lipid flip-flop event happens most frequently at the interface between symmetric and asymmetric DSPC domains. If both leaflets have identical area fraction of gel-phase, gel-phase domains are in registry and are static in comparison to the first state. The stability of these three DSPC domain distributions, the degree of registry observed, and the domain immobility have biological significance with regards to maintenance of lipid asymmetry in living cell membranes, communication between inner leaflet and outer leaflet, membrane adhesion, and raft mobility.
Figures



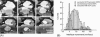
References
-
- Brown, D. A., and E. London. 2000. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275:17221–17224 [Review]. - PubMed
-
- Devaux, P. F., and R. Morris. 2004. Transmembrane asymmetry and lateral domains in biological membranes. Traffic. 5:241–246. - PubMed
-
- Devaux, P. F. 1991. Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 30:1163–1173. - PubMed
-
- Daleke, D. L. 2003. Regulation of transbilayer plasma membrane phospholipid asymmetry. J. Lipid Res. 44:233–242. - PubMed
-
- Kornberg, R. D., and H. M. McConnell. 1971. Inside-outside transitions of phospholipids in vesicle membranes. Biochemistry. 10:1111–1120. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

