A stochastic two-dimensional model of intercellular Ca2+ wave spread in glia
- PMID: 16214872
- PMCID: PMC1367023
- DOI: 10.1529/biophysj.105.064378
A stochastic two-dimensional model of intercellular Ca2+ wave spread in glia
Abstract
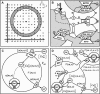
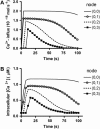
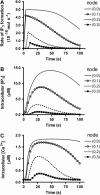
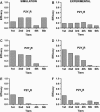
We describe a two-dimensional stochastic model of intercellular Ca(2+) wave (ICW) spread in glia that includes contributions of external stimuli, ionotropic and metabotropic P2 receptors, exo- and ecto-nucleotidases, second messengers, and gap junctions. In this model, an initial stimulus evokes ATP and UTP release from a single cell. Agonists diffuse and are degraded both in bulk solution and at cell surfaces. Ca(2+) elevation in individual cells is determined by bound agonist concentrations s and by number and features of P2 receptors summed with that generated by IP(3) diffusing through gap junction channels. Variability of ICWs is provided by randomly distributing a predetermined density of cells in a rectangular grid and by randomly selecting within intervals values characterizing the extracellular compartment, individual cells, and interconnections with neighboring cells. Variability intervals were obtained from experiments on astrocytoma cells transfected to express individual P2 receptors and/or the gap junction protein connexin43. The simulation program (available as Supplementary Material) permits individual alteration of ICW components, allowing comparison of simulations with data from cells expressing connexin43 and/or various P2 receptor subtypes. Such modeling is expected to be useful for testing phenomenological hypotheses and in understanding consequences of alteration of system components under experimental or pathological conditions.
Figures




Similar articles
-
Gap junction channels coordinate the propagation of intercellular Ca2+ signals generated by P2Y receptor activation.Glia. 2004 Nov 15;48(3):217-29. doi: 10.1002/glia.20071. Glia. 2004. PMID: 15390120 Free PMC article.
-
Pharmacological characterization of P2Y receptor subtypes on isolated tiger salamander Müller cells.Glia. 2003 Apr 15;42(2):149-59. doi: 10.1002/glia.10198. Glia. 2003. PMID: 12655599
-
The predominant mechanism of intercellular calcium wave propagation changes during long-term culture of human osteoblast-like cells.Cell Calcium. 2006 May;39(5):435-44. doi: 10.1016/j.ceca.2006.01.012. Epub 2006 Mar 20. Cell Calcium. 2006. PMID: 16545868
-
Purinergic signaling involved in Müller cell function in the mammalian retina.Prog Retin Eye Res. 2011 Sep;30(5):324-42. doi: 10.1016/j.preteyeres.2011.06.001. Epub 2011 Jun 14. Prog Retin Eye Res. 2011. PMID: 21689780 Review.
-
[Intra- and intercellular Ca(2+)-signal transduction].Verh K Acad Geneeskd Belg. 2000;62(6):501-63. Verh K Acad Geneeskd Belg. 2000. PMID: 11196579 Review. Dutch.
Cited by
-
Diffusion modeling of ATP signaling suggests a partially regenerative mechanism underlies astrocyte intercellular calcium waves.Front Neuroeng. 2008 Jul 17;1:1. doi: 10.3389/neuro.16.001.2008. eCollection 2008. Front Neuroeng. 2008. PMID: 18958241 Free PMC article.
-
Sparse short-distance connections enhance calcium wave propagation in a 3D model of astrocyte networks.Front Comput Neurosci. 2014 Apr 16;8:45. doi: 10.3389/fncom.2014.00045. eCollection 2014. Front Comput Neurosci. 2014. PMID: 24795613 Free PMC article.
-
Connexin43 and the brain transcriptome of newborn mice.Genomics. 2007 Jan;89(1):113-23. doi: 10.1016/j.ygeno.2006.09.007. Epub 2006 Oct 24. Genomics. 2007. PMID: 17064878 Free PMC article.
-
Estrogen Protects Neurotransmission Transcriptome During Status Epilepticus.Front Neurosci. 2018 Jun 20;12:332. doi: 10.3389/fnins.2018.00332. eCollection 2018. Front Neurosci. 2018. PMID: 29973860 Free PMC article.
-
Computational Models for Calcium-Mediated Astrocyte Functions.Front Comput Neurosci. 2018 Apr 4;12:14. doi: 10.3389/fncom.2018.00014. eCollection 2018. Front Comput Neurosci. 2018. PMID: 29670517 Free PMC article. Review.
References
-
- Scemes, E. 2000. Components of astrocytic intercellular calcium signaling. Mol. Neurobiol. 22:167–179. - PubMed
-
- Dermietzel, R., Y. Gao, E. Scemes, D. Vieira, M. Urban, M. Kremer, M. V. Bennett, and D. C. Spray. 2000. Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Res. Brain Res. Rev. 32:45–56. - PubMed
-
- Boitano, S., E. R. Dirksen, and M. J. Sanderson. 1992. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 258:292–295. - PubMed
-
- Leybaert, L., K. Paemeleire, A. Strahonja, and M. J. Sanderson. 1998. Inositol-trisphosphate-dependent intercellular calcium signaling in and between astrocytes and endothelial cells. Glia. 24:398–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

