Ca2+ changes the force sensitivity of the hair-cell transduction channel
- PMID: 16214875
- PMCID: PMC1367012
- DOI: 10.1529/biophysj.105.061226
Ca2+ changes the force sensitivity of the hair-cell transduction channel
Abstract
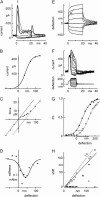
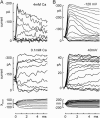
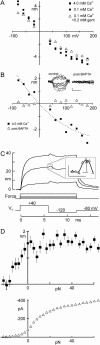
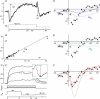
The mechanically gated transduction channels of vertebrate hair cells tend to close in approximately 1 ms after their activation by hair bundle deflection. This fast adaptation is correlated with a quick negative movement of the bundle (a "twitch"), which can exert force and may mediate an active mechanical amplification of sound stimuli in hearing organs. We used an optical trap to deflect bullfrog hair bundles and to measure bundle movement while controlling Ca(2+) entry with a voltage clamp. The twitch elicited by repolarization of the cell varied with force applied to the bundle, going to zero where channels were all open or closed. The force dependence is quantitatively consistent with a model in which a Ca(2+)-bound channel requires approximately 3 pN more force to open, and rules out other models for the site of Ca(2+) action. In addition, we characterized a faster, voltage-dependent "flick", which requires intact tip links but not current through transduction channels.
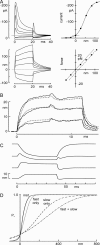
Figures



References
-
- Dallos, P., and D. Harris. 1978. Properties of auditory nerve responses in absence of outer hair cells. J. Neurophysiol. 41:365–383. - PubMed
-
- Brown, M. C., A. L. Nuttall, and R. I. Masta. 1983. Intracellular recordings from cochlear inner hair cells: effects of stimulation of the crossed olivocochlear efferents. Science. 222:69–72. - PubMed
-
- Brownell, W. E., C. R. Bader, D. Bertrand, and Y. de Ribaupierre. 1985. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 227:194–196. - PubMed
-
- Dallos, P., and B. N. Evans. 1995. High-frequency motility of outer hair cells and the cochlear amplifier. Science. 267:2006–2009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

