Rab11a and myosin Vb are required for bile canalicular formation in WIF-B9 cells
- PMID: 16214890
- PMCID: PMC1257697
- DOI: 10.1073/pnas.0503702102
Rab11a and myosin Vb are required for bile canalicular formation in WIF-B9 cells
Abstract
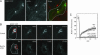
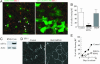
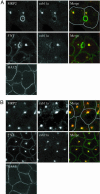
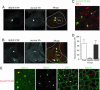
Hepatocytes polarize by forming functionally distinct sinusoidal (basolateral) and canalicular (apical) plasma membrane domains. Two distinct routes are used for delivery of membrane proteins to the canaliculus. Proteins having glycosylphosphatidylinositol anchors or single transmembrane domains are targeted to the sinusoidal plasma membrane from where they transcytose to the canalicular domain. In contrast, apical ATP-binding-cassette (ABC) transporters, which are required for energy-dependent biliary secretion of bile acids (ABCB11), phospholipids (ABCB4), and nonbile acid organic anions (ABCC2), lack initial residence in the basolateral plasma membrane and traffic directly from Golgi membranes to the canalicular membrane. While investigating mechanisms of apical targeting in WIF-B9 cells, a polarized hepatic epithelial cell line, we observed that rab11a is required for canalicular formation. Knockdown of rab11a or overexpression of the rab11a-GDP locked form prevented canalicular formation as did overexpression of the myosin Vb motorless tail domain. In WIF-B9 cells, which lack bile canaliculi, apical ABC transporters colocalized with transcytotic membrane proteins in rab11a-containing endosomes and, unlike the transcytotic markers, did not distribute to the plasma membrane. We propose that polarization of hepatocytes (i.e., canalicular biogenesis) requires recruitment of rab11a and myosin Vb to intracellular membranes that contain apical ABC transporters and transcytotic markers, permitting their targeting to the plasma membrane. In this model, polarization is initiated upon delivery of rab11a-myosin Vb-containing membranes to the surface, which causes plasma membrane at the site of delivery to differentiate into apical domain (bile canaliculus).
Figures
Similar articles
-
Intracellular trafficking of bile salt export pump (ABCB11) in polarized hepatic cells: constitutive cycling between the canalicular membrane and rab11-positive endosomes.Mol Biol Cell. 2004 Jul;15(7):3485-96. doi: 10.1091/mbc.e03-10-0737. Epub 2004 Apr 30. Mol Biol Cell. 2004. PMID: 15121884 Free PMC article.
-
Bile acid secretion and direct targeting of mdr1-green fluorescent protein from Golgi to the canalicular membrane in polarized WIF-B cells.J Cell Sci. 1999 Dec;112 ( Pt 24):4535-45. doi: 10.1242/jcs.112.24.4535. J Cell Sci. 1999. PMID: 10574703
-
Myosin Vb is required for trafficking of the cystic fibrosis transmembrane conductance regulator in Rab11a-specific apical recycling endosomes in polarized human airway epithelial cells.J Biol Chem. 2007 Aug 10;282(32):23725-36. doi: 10.1074/jbc.M608531200. Epub 2007 Apr 26. J Biol Chem. 2007. PMID: 17462998
-
Intracellular trafficking and regulation of canalicular ATP-binding cassette transporters.Semin Liver Dis. 2000;20(3):339-51. doi: 10.1055/s-2000-9388. Semin Liver Dis. 2000. PMID: 11076400 Review.
-
Mechanisms and functional features of polarized membrane traffic in epithelial and hepatic cells.Biochem J. 1998 Dec 1;336 ( Pt 2)(Pt 2):257-69. doi: 10.1042/bj3360257. Biochem J. 1998. PMID: 9820799 Free PMC article. Review.
Cited by
-
Extraintestinal manifestations in an infant with microvillus inclusion disease: complications or features of the disease?Eur J Pediatr. 2013 Sep;172(9):1271-5. doi: 10.1007/s00431-013-1948-0. Epub 2013 Jan 25. Eur J Pediatr. 2013. PMID: 23354788
-
Rab GTPase-Myo5B complexes control membrane recycling and epithelial polarization.Proc Natl Acad Sci U S A. 2011 Feb 15;108(7):2789-94. doi: 10.1073/pnas.1010754108. Epub 2011 Jan 31. Proc Natl Acad Sci U S A. 2011. PMID: 21282656 Free PMC article.
-
Interplay between P-Glycoprotein Expression and Resistance to Endoplasmic Reticulum Stressors.Molecules. 2018 Feb 6;23(2):337. doi: 10.3390/molecules23020337. Molecules. 2018. PMID: 29415493 Free PMC article. Review.
-
A Rab11a-enriched subapical membrane compartment regulates a cytoskeleton-dependent transcytotic pathway in secretory epithelial cells of the lacrimal gland.J Cell Sci. 2011 Oct 15;124(Pt 20):3503-14. doi: 10.1242/jcs.088906. Epub 2011 Oct 7. J Cell Sci. 2011. PMID: 21984810 Free PMC article.
-
The cell biology of the hepatocyte: A membrane trafficking machine.J Cell Biol. 2019 Jul 1;218(7):2096-2112. doi: 10.1083/jcb.201903090. Epub 2019 Jun 14. J Cell Biol. 2019. PMID: 31201265 Free PMC article. Review.
References
-
- Gerloff, T., Stieger, B., Hagenbuch, B., Madon, J., Landmann, L., Roth, J., Hofmann, A. F. & Meier, P. J. (1998) J. Biol. Chem. 273, 10046–10050. - PubMed
-
- Smit, J. J., Schinkel, A. H., Oude Elferink, R. P., Groen, A. K., Wagenaar, E., van Deemter, L., Mol, C. A., Ottenhoff, R., van der Lugt, N. M., van Roon, M. A., et al. (1993) Cell 75, 451–462. - PubMed
-
- Berge, K. E., Tian, H., Graf, G. A., Yu, L., Grishin, N. V., Schultz, J., Kwiterovich, P., Shan, B., Barnes, R. & Hobbs, H. H. (2000) Science 290, 1771–1775. - PubMed
-
- Buchler, M., Konig, J., Brom, M., Kartenbeck, J., Spring, H., Horie, T. & Keppler, D. (1996) J. Biol. Chem. 271, 15091–15098. - PubMed
-
- Paulusma, C. C., Bosma, P. J., Zaman, G. J., Bakker, C. T., Otter, M., Scheffer, G. L., Scheper, R. J., Borst, P. & Oude Elferink, R. P. (1996) Science 271, 1126–1128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

