A phase II trial of vaccination with autologous, tumor-derived heat-shock protein peptide complexes Gp96, in combination with GM-CSF and interferon-alpha in metastatic melanoma patients
- PMID: 16215718
- PMCID: PMC11031093
- DOI: 10.1007/s00262-005-0084-8
A phase II trial of vaccination with autologous, tumor-derived heat-shock protein peptide complexes Gp96, in combination with GM-CSF and interferon-alpha in metastatic melanoma patients
Abstract
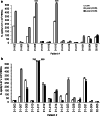
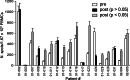
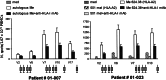
The aim of this study was to determine the immunogenicity and antitumor activity of autologous, tumor-derived heat shock protein gp96-peptide complex vaccine (HSPPC-96; Oncophage given with GM-CSF and IFN-alpha in pre-treated metastatic (AJCC stage IV) melanoma patients. Patients underwent surgical resection of metastatic lesions for HSPPC-96 production. HSPPC-96 was administered subcutaneously (s.c.) in four weekly intervals (first cycle). Patients with more available vaccine and absence of progressive disease received four additional injections in 2-week intervals (second cycle) or more. GM-CSF was given s.c. at the same site at days -1, 0 and +1, while IFN-alpha (3 MU) was administered s.c. at a different site at days +4 and +6. Antigen-specific anti-melanoma T and NK lymphocyte response was assessed by enzyme-linked immunospot assay on peripheral blood mononuclear cells obtained before and after vaccination. Thirty-eight patients were enrolled, 20 received at least four injections (one cycle) of HSPPC-96 and were considered assessable. Toxicity was mild and most treatment-related adverse events were local erythema and induration at the injection site. Patients receiving at least four injections of HSPPC-96 were considered evaluable for clinical response: of the 18 patients with measurable disease post surgery, 11 showed stable disease (SD). The ELISPOT assay revealed an increased class I HLA-restricted T and NK cell-mediated post-vaccination response in 5 out of 17 and 12 out of the 18 patients tested, respectively. Four of the five class I HLA-restricted T cell responses fall in the group of SD patients. Vaccination with autologous HSPPC-96 together with GM-CSF and IFN-alpha is feasible and accompanied by mild local and systemic toxicity. Both tumor-specific T cell-mediated and NK cell responses were generated in a proportion of patients. Clinical activity was limited to SD. However, both immunological and clinical responses were not improved as compared with those recorded in a previous study investigating HSPPC-96 monotherapy.
Figures
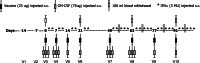

References
-
- Belli F, Testori A, Rivoltini L, Maio M, Andreola G, Sertoli MR, Gallino P, Piris A, Cattelan A, Lazzari I, Carrabba M, Scita G, et al. Vaccination of metastatic melanoma patients with autologous tumor-derived heat shock protein gp96-peptide complexes: Clinical and immunologic findings. J Clin Oncol. 2002;20:4169–4180. doi: 10.1200/JCO.2002.09.134. - DOI - PubMed
-
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte-macrophage colony stimulating factor. J Exp Med. 1992;176:1693–1699. doi: 10.1084/jem.176.6.1693. - DOI - PMC - PubMed
-
- Soiffer R, Lynch T, Mihm M, Jung K, Rhuda C, Schmollinger JC, Hodi FS, Liebster L, Lam P, Mentzer S, Singer S, Tanabe KK, et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage-colony stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci USA. 1998;95:13141–13146. doi: 10.1073/pnas.95.22.13141. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

