Rhinovirus infection induces cytotoxicity and delays wound healing in bronchial epithelial cells
- PMID: 16216126
- PMCID: PMC1283981
- DOI: 10.1186/1465-9921-6-114
Rhinovirus infection induces cytotoxicity and delays wound healing in bronchial epithelial cells
Abstract
Background: Human rhinoviruses (RV), the most common triggers of acute asthma exacerbations, are considered not cytotoxic to the bronchial epithelium. Recent observations, however, have questioned this knowledge. The aim of this study was to evaluate the ability of RV to induce epithelial cytotoxicity and affect epithelial repair in-vitro.
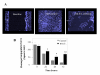
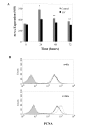
Methods: Monolayers of BEAS-2B bronchial epithelial cells, seeded at different densities were exposed to RV serotypes 1b, 5, 7, 9, 14, 16. Cytotoxicity was assessed chromatometrically. Epithelial monolayers were mechanically wounded, exposed or not to RV and the repopulation of the damaged area was assessed by image analysis. Finally epithelial cell proliferation was assessed by quantitation of proliferating cell nuclear antigen (PCNA) by flow cytometry.
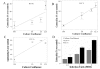
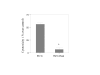
Results: RV1b, RV5, RV7, RV14 and RV16 were able to induce considerable epithelial cytotoxicity, more pronounced in less dense cultures, in a cell-density and dose-dependent manner. RV9 was not cytotoxic. Furthermore, RV infection diminished the self-repair capacity of bronchial epithelial cells and reduced cell proliferation.
Conclusion: RV-induced epithelial cytotoxicity may become considerable in already compromised epithelium, such as in the case of asthma. The RV-induced impairment on epithelial proliferation and self-repair capacity may contribute to the development of airway remodeling.
Figures

References
-
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161(5):1720–1745. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

